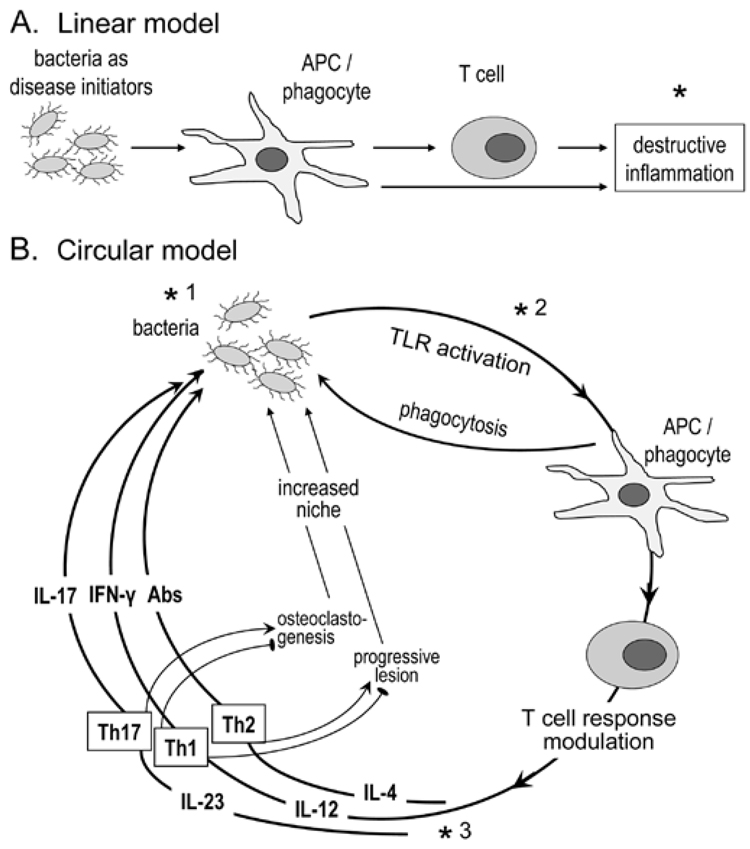
Figure 2.
Concepts in periodontal disease pathogenesis. In the linear model (A), the bacteria are seen as initiators of the inflammatory process. Disease progression depends exclusively on the host response, which offers a target (*) for therapeutic intervention (e.g., anti-inflammatory agents) (Salvi and Lang, 2005). The circular model (B) contends that bacteria are necessary for both initiation and progression of the disease, by constantly shaping the T-cell response through differential TLR-mediated activation of APC and secreted cytokines. The innate and adaptive host response, in turn, determines the fate of the infection, and consequently whether inflammation will progress to disease or be controlled. Although the role of the individual effector Th subsets is debatable, Th2 cells have been associated with non-protective antibody responses and progressive periodontal lesions, and Th1 with stable lesions (indicated by an inhibitory sign against disease progression;  ) (Gemmell et al., 2002). Th1 cells are thought to protect through IL-12/IFNγ-stimulated cell-mediated immunity (Alayan et al., 2007b; Hajishengallis et al., 2007) and by inhibition of osteoclastogenesis (Gowen et al., 1986; Horwood et al., 2001). In contrast, Th17 cells have been implicated as a specialized osteoclastogenic subset that links T-cell activation to bone loss (Sato et al., 2006). Contrary to this potentially destructive role, IL-17 produced by this subset has been associated with neutrophil-mediated control of the bacterial challenge (Yu et al., 2007). Periodontal bone loss and soft-tissue alterations offer new niches for colonization (generation of deep periodontal pockets) and thus facilitate bacterial overgrowth; this presents increased challenge and reinforces the circular process or, metaphorically, the ongoing vicious cycle. This model offers three types of targets for therapeutic intervention: antimicrobial (*1), TLR-based modulation of APC responses (*2), and cytokine-based control of T-cell activation (*3).
) (Gemmell et al., 2002). Th1 cells are thought to protect through IL-12/IFNγ-stimulated cell-mediated immunity (Alayan et al., 2007b; Hajishengallis et al., 2007) and by inhibition of osteoclastogenesis (Gowen et al., 1986; Horwood et al., 2001). In contrast, Th17 cells have been implicated as a specialized osteoclastogenic subset that links T-cell activation to bone loss (Sato et al., 2006). Contrary to this potentially destructive role, IL-17 produced by this subset has been associated with neutrophil-mediated control of the bacterial challenge (Yu et al., 2007). Periodontal bone loss and soft-tissue alterations offer new niches for colonization (generation of deep periodontal pockets) and thus facilitate bacterial overgrowth; this presents increased challenge and reinforces the circular process or, metaphorically, the ongoing vicious cycle. This model offers three types of targets for therapeutic intervention: antimicrobial (*1), TLR-based modulation of APC responses (*2), and cytokine-based control of T-cell activation (*3).