Figure 2.
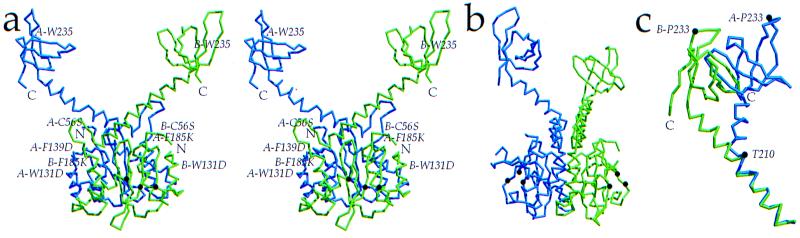
Structure of HIV-1 IN52–288. (a) Stereoview of the HIV-1 IN52–288 dimer, composed of monomer A (blue) and monomer B (green). Monomer B catalytic residues D64, D116, and E152 are indicated (brown dots), and the N and C termini of each monomer are labeled. Immunologically critical residue W235 is located on the surface. Mutated residues C56S, W131D, F139D, and F185K are indicated, except for C280S, which is disordered. (b) The HIV-1 IN52–288 dimer rotated by 90° with respect to a. Catalytic residues are highlighted in brown. (c) Alignment of residues 195–210 in α6 demonstrates the kink at T210 that creates a ≈90° rotation of the C-terminal domains relative to one another as illustrated by the position of P233. Figure was generated by molscript (44) and raster3d (45).