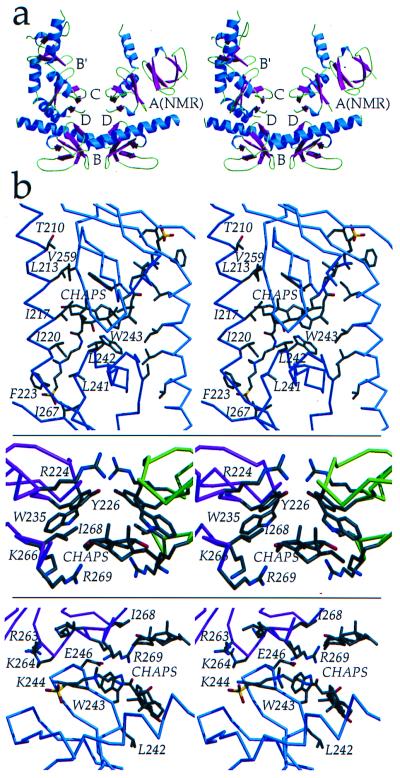
Figure 5.
SH3–SH3 interactions. (a) The C-terminal domains within the IN52–288 dimer are 55 Å apart, but four different dimer-dimer contacts involving interactions between adjacent C-terminal domains, interfaces B, B′, C, and D, are found within the crystal. All four of these interfaces differ from interface A, which is found in the NMR structure of isolated C-terminal domains. Buried molecular surface areas for the interfaces are: B = 1,695 Å (2), B′ = 2,589 Å (2), C = 697 Å (2), D = 764 Å (2), and A(NMR) = 660 Å (2). β-strands (magenta), α-helices (blue), and loops (green) are color coded. (b) Interactions between adjacent C-terminal domains and protein-detergent (CHAPS) interactions are shown. (Top) Interface B, in an orientation rotated 90° relative to that in a. (Middle) Interface C. (Bottom) Interface D. Interfaces C and D are in an identical orientation as in a. Figure was generated by using molscript (44) and raster3d (45). Surface area was calculated by using surface (48).