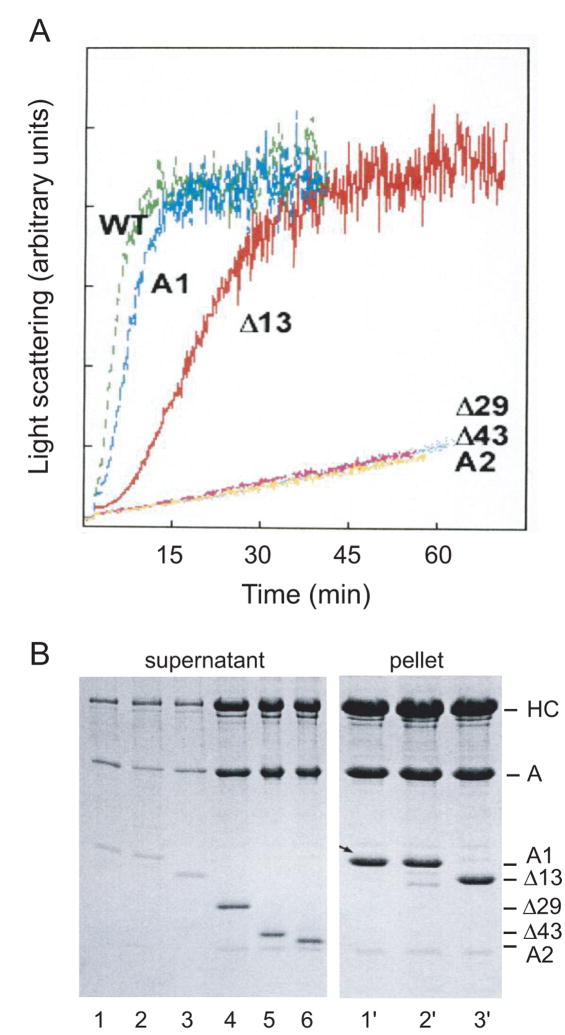
Figure 2.
(A) Time course of polymerization of G-actin by S1 reconstituted with ELC mutants (see Fig. 1). Light scattering was monitored at 325 nm in the ISS spectrofluorometer. S1 and G-actin, each at a concentration of 5μM, were in 5 mM Tris, 0.2 mM CaCl2, pH 8. S1 (WT) accelerated polymerization of G-actin even slightly more than native S1(A1), whereas deletion of the actin binding site, along with the ProAla region (Δ29), slowed polymerization to the rate observed for A2 or Δ43. The Δ13 mutant, which had two Arg residues at the N-terminus, did accelerate assembly of actin, although at a slower rate. Another mutant (Δ14), with only one Arg, had a very slight effect on assembly (data not shown; see similar results by cross-linking in Andreev et al., 199913). (B) Samples taken from the light scattering measurements after one hr were centrifuged, and the supernatants and pellets were analyzed by SDS-PAGE. As expected, S1 containing native A1, wild-type LC1, and Δ13 was found in the pellet (lanes 1′, 2′, and 3′), whereas S1 containing Δ29, Δ43, and A2 remained in the supernatant (lanes 4, 5, and 6). The amount of protein in the pellets for the S1 with A1 truncations that did not polymerize G-actin was very minor, and samples for lanes 4′– 6′ are omitted.