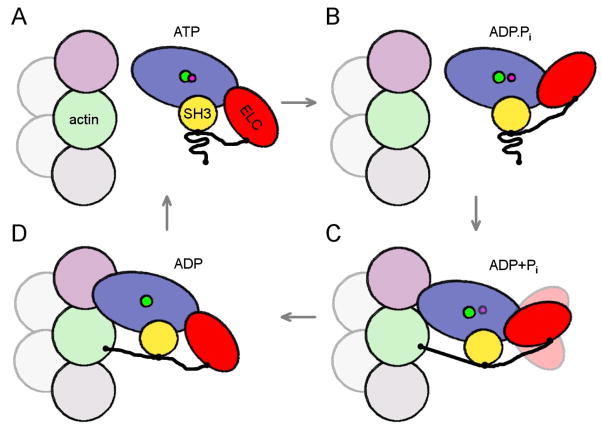
Figure 5.
Schematic drawing of proposed events in the myosin II ATPase cycle. Motor domain is shown in blue, ELC in red with extension as thick black line, SH3 domain in yellow. The subunit corresponding to the upper actin in Fig 1C is shown in pink, the lower actin in Fig 1C in green. An additional actin subunit along the long-pitch helix is shown in gray and two subunits along the opposite long-pitch helix are shown in light-gray. The pointed end of the filament is at the top of the figure. (A, B) In the detached states, ATP, ADP.Pi, the proline-rich region of the ELC extension is bound to the SH3 domain, and does not interact with actin. (C) After initial weak binding to actin, the cross-bridge progresses to a stronger force-generating state (ADP+Pi), with the ELC extension still attached to the SH3 domain, but with the N-terminus of the extension now weakly bound to the lower actin. If the lower actin is occupied by another myosin molecule, as in our study, the ELC extension can not bind to it. (D) Strong binding to actin induces the release of Pi and the N-terminus of the ELC extension stabilizes the lever arm by binding to the C-terminus of the lower actin.