Table 1.
Ba ENR inhibition and antibacterial activity for modifications of ring A.
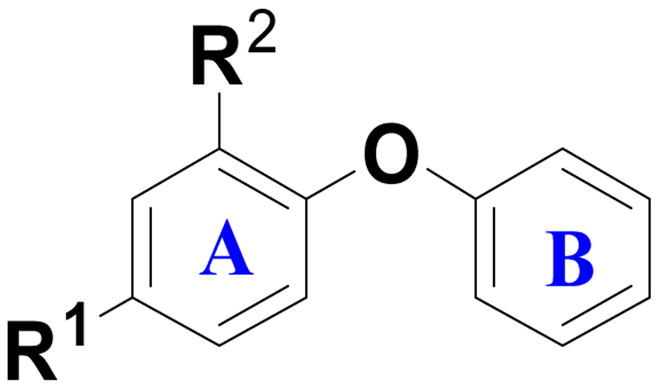
| ||||
|---|---|---|---|---|
| Compound | R1 | R2 | IC50 (μM) or % inhibition at 1μM | MIC[b] (μg/mL) |
| Triclosan | 0.6 ± 0.0 | 3.1 | ||
| 52 | Cl |
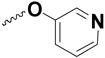
|
0.5% | NT |
| 53 | Cl | OMe | 0% | NT |
| 54 | Cl | NH2 | 4.4% | NT |
| 14 | Cl | CO2H | 0% | NT |
| 16 | Cl | CH2OH | 1.8% | NT |
| 17 | Cl | CONH2 | 0% | NT |
| 55 | Cl | OH | 0.5 ± 0.1 | 32 |
| 1 | OH | OH | 6.3 ± 0.4 | 64 |
| 2 | NO2 | OH | >50 | 5.8 |
| 26 | CH2CH(OH)CH2OH | OH | 9.8% | >104 |
| 28 | CH2OH | OH | 5.0% | 43 |
| 29 | CH2NHCH(CH3)CH2CH2CH3 | OH | 0% | >109 |
| 30 | CH2–(1–piperidine) | OH | 0% | >113 |
| 31 | CH2NHCH2Ph | OH | 7.9% | >122 |
| 27 | n-propyl | OH | >0.8[a] | 22.8 |
Saturation with inhibitor was not obtained over the concentration range tested. The percent inhibition of BaENR showed a linear response to increasing inhibitor concentrations.
MIC values are against ΔANR B. anthracis. NT = Not tested.
