Figure 1.
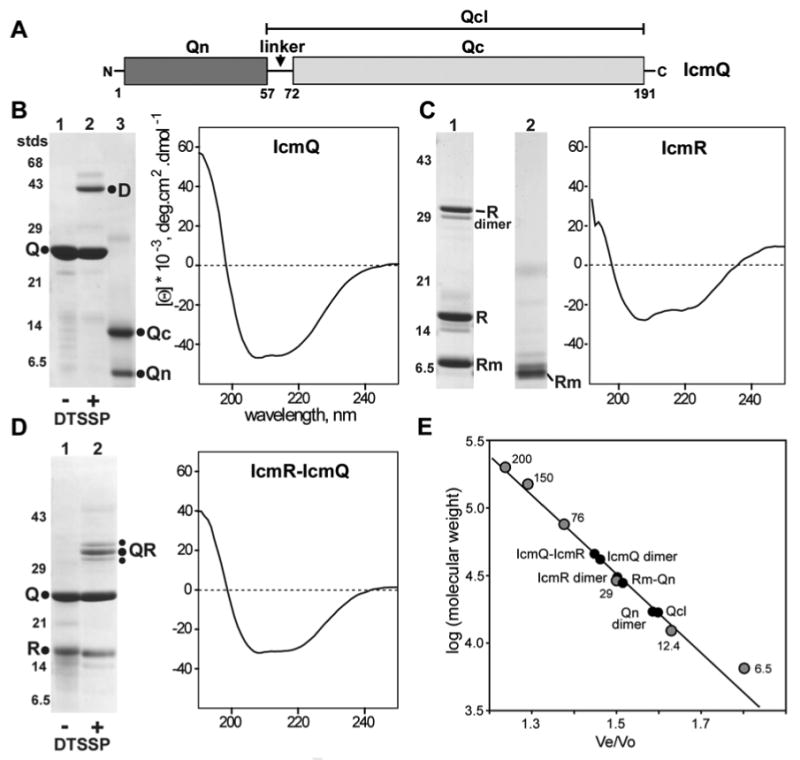
Purification and characterization of IcmQ, IcmR and IcmR-IcmQ.
A. A domain diagram is shown for IcmQ.
B. (left) Purified IcmQ is shown on an SDS gel (lane 1). Crosslinking with DTSSP reveals an IcmQ dimer (lane 2). Trypsinization of IcmQ overnight at a 1:1000 ratio (wt/wt) generates Qn and Qc domains (lane 3). (right) A CD spectrum of IcmQ shows that the protein is mostly α-helical.
C. (left) Purified IcmR is shown in lane 1 and the moderately trypsin resitant product is shown in lane 2. (right) IcmR is strongly α-helical based on its CD spectrum.
D. (left) Purified IcmR-IcmQ complex is shown on an SDS gel (lane 1) and DTSSP crosslinking reveals a simple heterodimer (lane 2). (right) IcmR-IcmQ retains the α-helicity of its components, based on a CD spectrum.
E. Molecular weights of IcmQ, IcmQ-domains, IcmR and their complexes were determined on a calibrated Superose-12 HR column. A plot of the log of molecular mass versus elution position is shown for standards and the purified proteins (also see S_Table 1).
