Abstract
Transitive inference (TI) is the ability to infer the relationship between items (e.g., A>C) after having learned a set of premise pairs (e.g., A>B and B>C). Previous studies in humans have identified a distributed neural network, including cortex, hippocampus, and thalamus, during TI judgments. We studied two aspects of TI using fMRI of subjects who had acquired the 6-item sequence (A>B>C>D>E>F) of visual stimuli. First, the identification of novel pairs not containing end items (i.e., B>D, C>E, B>E) was associated with greater left hippocampal activation when compared to the identification of novel pairs containing end items A and F. This demonstrates that the identification of stimulus pairs requiring the flexible representation of a sequence is associated with hippocampal activation.
Second, for the three novel pairs devoid of end items we found greater right hippocampal activation for pairs B>D and C>E compared with pair B>E. This indicates that TI decisions on pairs derived from more adjacent items in the sequence are associated with greater hippocampal activation. Hippocampal activation thus scales with the degree of relational processing necessary for TI judgments.
Both findings confirm a role of the hippocampus in transitive inference in humans.
Keywords: Relational memory, prefrontal cortex, insula, precuneus, thalamus, symbolic distance
1. Introduction
The ability of transitive inference has attracted great interest in the study of human and animal cognition (Piaget, 1928; McGonigle and Chalmers, 1986; Dusek and Eichenbaum, 1997; Acuna et al., 2002a; Van Elzakker et al., 2003; Buckmaster et al., 2004; Guillermo Paz-y-Mino et al., 2004; Frank et al., 2005; Ellenbogen et al., 2007; Libben and Titone, 2008). After learning a set of overlapping premise pairs (e.g., A>B, B>C, C>D, D>E), subjects are tested for their ability to identify the correct order in novel pairs that do contain end items (e.g., AE) or that do not contain end items (i.e., BD for a 5-item sequence). Using this experimental design, the capacity for transitive inference has been demonstrated in birds (von Fersen et al., 1991; Bond et al., 2003), rodents (Davis, 1992; Dusek and Eichenbaum, 1997; Van Elzakker et al., 2003), monkeys (McGonigle and Chalmers, 1986; Buckmaster et al., 2004) and humans (Greene et al., 2001; Martin and Alsop, 2004), with several similarities between animal and human performance (Colombo and Frost, 2001).
In both rats (Dusek and Eichenbaum, 1997) and monkeys (Buckmaster et al., 2004), disconnection of the hippocampus from either its cortical or subcortical pathway results in the animals’ inability to correctly chose B over D while their ability to chose A over E is spared. This finding has been interpreted as key evidence for the flexible relational memory account of the hippocampus in animals (McGonigle and Chalmers, 1986; Eichenbaum, 1992; Squire, 1992; Cohen and Eichenbaum, 1993; Dusek and Eichenbaum, 1997; Burgess et al., 2002). In this account, hippocampal function is closely related to all declarative memory, but is especially crucial for relational learning and flexible use of memory (Eichenbaum, 2004). In contrast, the excitatory strength/value transfer account posits that performance on transitive inferences is guided by the absolute excitatory strength that each stimulus acquires during training, rather than the flexible manipulation of the sequence representation (von Fersen et al., 1991; Wynne, 1998; Frank et al., 2003; Van Elzakker et al., 2003).
In a previous functional magnetic resonance imaging (fMRI) study (Heckers et al., 2004), we studied the neural basis of transitive inference in subjects who had acquired the flexible representation of a 5-item sequence (A>B>C>D>E). We demonstrated a role of the hippocampus in blocked trials that did (e.g., AC) and did not (BD) contain end items. This finding was interpreted as further evidence for the relational memory account of the hippocampus (Eichenbaum, 2004; Rapp, 2004). However, only novel pairs not containing end items necessitate the flexible representation of a sequence. We were therefore unable to prove a role of the hippocampus specifically for those transitive inference trials that can be solved only with reference to an underlying sequence.
Here we report the results of an event-related fMRI experiment after the acquisition of a 6-item sequence (A>B>C>D>E>F). This design allowed us to directly compare transitive inference trials with and without end items. We hypothesized that the identification of the three novel pairs not containing end items (BD, BE, CE) would be associated with greater hippocampal activity when compared with transitive inference judgments containing end items, which could be solved relying on the end item with an unambiguous reward history (i.e., A was always rewarded, F was never rewarded.)
The experimental design employed here also allowed us to study the neural basis of the symbolic distance effect (SDE). SDE refers to the decrease in reaction time on inference decisions the further apart the two items are on the relational continuum (Frank et al.,; Rapp et al., 1996; Acuna et al., 2002a; Bond et al., 2003; Frank et al., 2005).
We tested for the SDE in the three novel pairs not including end items: the pairs BD and CE have a symbolic distance of one (referred to as: SDE1), whereas the pair BE has a symbolic distance of two (referred to as: SDE2).
As suggested by SDE, inference decisions on pairs with a symbolic distance of 1 require greater manipulation of the sequence relationships than those on pairs with a symbolic distance of 2. Therefore, we hypothesized that this contrast would reveal significant hippocampal activation in humans. Thus we predicted that neuroimaging of the SDE would support the relational memory account of transitive inference.
In addition to the hippocampus, a number of cortical regions have been shown to be recruited during TI judgments: the pre-supplementary motor area (pre-SMA), parietal, prefrontal, and inferior temporal cortices, the insula, anterior cingulate cortex, precuneus and thalamus (Acuna et al., 2002b; Heckers et al., 2004). We hypothesized that this network of brain regions would also be more active in our comparisons of non end-item and end-item TI judgments, and of symbolic distances of 1 and 2.
2. Materials and methods
2.1. Subjects
We studied 13 healthy subjects (10 male and 3 female, aged 22 to 38, mean age = 26.9, mean estimated verbal IQ (Blair and Spreen, 1989) = 119.1), who gave informed consent in a manner approved by the institutional review board of the Massachusetts General Hospital. None of the subjects had a history of major medical, neurological, or psychiatric illness.
2.2. Stimuli and paradigm
2.2.1. Stimuli
Sixteen visually distinctive pattern fills were selected from the CorelDraw graphical package, as used in our previous TI study (Heckers et al., 2004). Six of these pattern fills were used to create a set of overlapping pairs of either pentagon or ellipsoid shape for each participant as described in our previous work (Heckers et al., 2004). To avoid bias associated with particular object shape and/or pattern, we rotated the position of the fills within the set for each participant and the two shapes across all subjects (seven subjects saw pentagons and six subjects ellipses). The five pairs made out of the six stimuli A through F were denoted AB, BC, CD, DE and EF.
2.2.2. Training prior to scanning
The training was divided into three stages. Participants were first informed that they would be trained on pairs of visual patterns and that one of the two patterns in the pair would be the “winner”.
In Stage 1, participants saw a preview of the five pairs on a computer screen. Each pair was viewed for ten seconds. They were told that the pattern associated with a smiley face (☺) on the screen was the superior one and asked to commit the pairs along with the winning pattern to memory.
In Stage 2, participants were trained on these pairs to reinforce their memory of the pairs and the winning patterns in each pair. They were informed that they would see the pairs of patterns on the computer screen and that one pattern in each pair would always hide a smiley face. The pattern hiding the smiley face would be the same one that was the superior one in each pair they saw in the preview. Left/right position of individual patterns for each pair was counterbalanced, and participants indicated their response by pressing “1” for the stimulus on the left and “2” for the stimulus on the right. When participants made a correct choice during training, the selected visual pattern would move to reveal the smiley face reinforcement. This stage of training was untimed; the patterns remained on the screen until the subject made a choice.
When participants made an incorrect choice, the selected visual pattern would move but the smiley face would not appear. Stage 2 consisted of two training blocks. The first training block consisted of 90 trials that were front-loaded so that the first three pairs from the total of five pairs would occur more often than the remaining two pairs. In this block, they saw 22 instances of the first three pairs (AB, BC, and CD) and 12 instances of the remaining two pairs (DE and EF). The second training block consisted of 80 trials that were back-loaded with the last two pairs. In this block, they saw 12 instances of the first three pairs and 22 instances of the remaining two pairs.
In Stage 3, participants were presented with the stimulus pairs on the screen and had to indicate the winning pattern without receiving feedback. In this training period, subjects saw thirty randomly ordered pairs in total, six for each pair. The subjects had three seconds to respond to each pair. The first purpose of this test run was to help subjects get accustomed to the testing conditions in the scanner (i.e., the random and paced (3 sec) presentation of stimulus pairs without any feedback). The second purpose was to apply the criterion of 90% or greater accuracy across the stimulus pairs. All thirteen subjects passed this criterion and performed the subsequent tests in the scanner.
Overall, this method of training ensured that participants would not only be able to learn the correct response for each pairing but would also be likely to hierarchically encode the overlapping stimulus set. Previous work suggested that the initial front loading of pairs was necessary for healthy participants to respond correctly during the transitive inference trials devoid of end items, i.e., BD, CE and BE (Heckers et al., 2004; Titone et al., 2004).
2.2.3. Test during fMRI scan
Subjects participated in two fMRI sessions, each comprising 96 randomly ordered pairs from the set of the overlapping stimuli. Of these, there were 30 instances of the previously seen overlapping pairs (referred to as: S pairs), 36 novel pairs requiring TI and containing an end item (referred to as: ETI pairs) and 30 novel pairs requiring TI but devoid of end items (referred to as: NETI pairs). For the 30 S pairs, subjects saw six instances of each of these pairs (AB, BC, CD, DE and EF). For the 36 ETI pairs, subjects saw five instances of each of these pairs (AD, AE, AC, BF, CF and DF). For the 36 NETI pairs, subjects saw ten instances of each of these pairs (BD, CE and BE). The relative (left vs. right) position of the two patterns was counterbalanced for each pair. For each trial, subjects were instructed to indicate the pattern they considered superior by pressing the appropriate button (left or right) based on the preview and the training sessions. Each pair would remain on the screen for three seconds before the screen was refreshed and a new pair came on. No feedback was supplied during the scanning. Our design was devised to test for behavioral and neural correlates of two effects, inference (NETI vs. ETI) and symbolic distance (SDE1 vs. SDE2).
The test was followed by a brief questionnaire administered outside of the scanner. The questionnaire was designed to detect whether each subject possessed explicit awareness of the sequence hierarchy.
2.3. Functional imaging
Subjects were scanned in a Siemens 3.0 Tesla Trio high-speed echo-planar imaging device (Munich, Germany). Subjects lay on a padded scanner bed in a dimly illuminated room, wearing ear plugs. Foam padding was used to restrict head movement. Stimuli were generated using Presentation software (Neurobehavioral Systems) on a personal computer, projected onto a screen, and viewed by the subjects via a tilted mirror placed in front of their eyes.
Functional scanning began with an initial sagittal localizer scan. A GRE scan with successive flip angles was then performed to allow for automatic alignment of anatomical images. A three-dimensional MPRAGE anatomical image was then obtained and automatically positioned within a common anatomical database based on the anterior commisure-posterior commisure (AC-PC) axis. The two functional series lasted 8 minutes and 44 seconds each. The first ten seconds of each series were discarded to allow equilibration of the MRI signal. During the remaining time of each series, 257 BOLD gradient-echo EPI functional brain images were collected (TE/TR = 28/2000 ms; 34 axial slices, parallel to the AC-PC line and starting anteriorly at the frontal pole, interleaved order, 3 mm slice thickness, voxel size 3.1×3.1×3 mm, FOV = 200 mm, flip angle = 90 degrees), to capture 96 trials lasting three seconds each. The inter-trial period (three seconds) was different from the scanner repetition time (TR = 2 seconds) to allow jitter in the design and efficient sampling of the haemodynamic response curve for each stimulus type.
2.4. Data analysis
2.4.1. Behavioral data
Behavioral data for the novel pairs were analyzed with the aim to assess the effect of inference and symbolic distance. We used a paired sample t-test to compare, within each subject, the reaction times for 1) NETI (BD, BE and CE) and ETI (AC, AD, AE, DF, CF and BF) trials and 2) NETI trials with symbolic distance of one (BD and CE) and two (BE).
2.4.2. Whole-brain voxel-based analysis of the functional neuroimaging data
fMRI data from each subject were analyzed using the Statistical Parametric Mapping SPM2 package (Wellcome Department of Imaging Neuroscience, London, UK). Pre-processing was carried out using this package. First, slice timing correction using sinc interpolation was applied to account for differences in acquisition time of the individual slices. Functional images were then realigned to correct for head motion during the scanning and a mean functional image was created. The anatomical image of each subject was then coregistered with the subject’s mean functional image. The coregistered anatomical image was normalized to a T1-weighted anatomical template in the stereotactic MNI coordinates. The functional images were subsequently normalized using the identical parameters and smoothed with a three-dimensional 8-mm Gaussian kernel to eliminate spatial noise and allow the application of the Gaussian Random Field Theory in statistical analysis.
A design matrix was then created to allow the application of the General Linear Model (GLM) to the functional data. Different instances of each pair were modeled together as a unique condition. The onset times of these conditions were entered into the GLM and convolved with the haemodynamic response function and its time and dispersion derivatives to model the haemodynamic response to each trial type. A high-pass filter of 128 s was used to remove time-dependent drift in the functional data and the data were corrected for serial correlations.
Functional neuroimaging data were analyzed with the aim to assess the effects of transitive inference type (NETI vs. ETI) and symbolic distance (SDE1 vs. SDE2). The contrast images [NETI-ETI] and [SDE1-SDE2] were therefore formed for each subject. The individual contrast images were then entered as random effects into a one-sample t-test to detect significant differences common to all subjects.
Given the a priori hypothesis of the involvement of MTL and the extra-MTL network in TI, the significance level of P<0.005, uncorrected for multiple comparisons, was used to assess the statistical significance for the main effect of inference and symbolic distance in these regions. For other brain regions with no a priori hypothesis, we used the P<0.05 threshold, corrected for multiple comparisons using the family-wise error control in SPM2. All of the resulting activation maps were examined for significant differences at a voxel extent threshold of five voxels. The resulting activation maps were overlaid on the mean anatomical image of the subjects whose contrast images were used for imaging analysis. The peak activations were identified and their Talairach coordinates and peak Z-scores were noted.
2.4.3. Region-of-interest analysis of the functional neuroimaging data
The whole-brain voxel-based analysis described above was supplemented by an anatomically guided ROI analysis. This analysis was motivated by the fact that the whole-brain normalization procedure results in an imperfect overlap between brain structures of different subjects. This morphing is particularly problematic in the hippocampus given its high degree of anatomical variability across the population (Pruessner et al., 2000; Miller et al., 2005).
The hippocampus was outlined bilaterally on every subject’s anatomical image in native space using guidelines described elsewhere (Pruessner et al., 2000; DeWitt et al., 2002). This anatomical ROI was then resampled to the same resolution as the native space BOLD functional images that had been previously realigned and coregistered with the native space anatomical image. The resampled anatomical ROI image was then used as a mask to create partial brain functional images, with signal intensity values outside the hippocampal mask set to zero. These images were subsequently smoothed using a three-dimensional 3-mm Gaussian kernel to increase detection sensitivity.
The design matrix from the whole-brain analysis was used with these partial brain volumes to apply GLM and extract parameter estimates for the conditions of interest as described above. As for the whole-brain analysis, the contrast images [NETI-ETI] and [SDE1-SDE2] were obtained for each subject bilaterally. Each hippocampus was then divided into nine segments longitudinally along the y (anterior-posterior) axis and average contrast values were computed for each of the nine segments bilaterally. Because of concerns about signal loss in the anterior portion of the MTL, we identified hippocampal voxels with signal intensity below 1/6 of the mean hippocampal intensity. These voxels, exhibiting a pronounced signal loss, were excluded from the analysis.
Analogously to the group analysis for the whole-brain data, the contrast values from each segment were then entered as random effects into a one-sample t-test to detect significant activations common to all subjects. In each case, a one-sided t-test (based on our hypothesis that the hippocampus would be more active for NETI than ETI and SDE1 than SDE2) with a P-value threshold of 0.05 was used. Finally, we performed a regression analysis within each subject to determine whether signal loss affected the estimated contrast values.
3. Results
3.1. Behavioral data
Accuracy did not differ between the four trial types (mean ± standard deviation: novel pairs containing end items, 99.3 ± 0.5%; novel pairs not containing end items, 99.6 ± 0.9%; novel pairs not containing end items with a symbolic distance of 1, 99.5 ± 0.8%; novel pairs not containing end items with a symbolic distance of 2, 99.6 ± 1.3%).
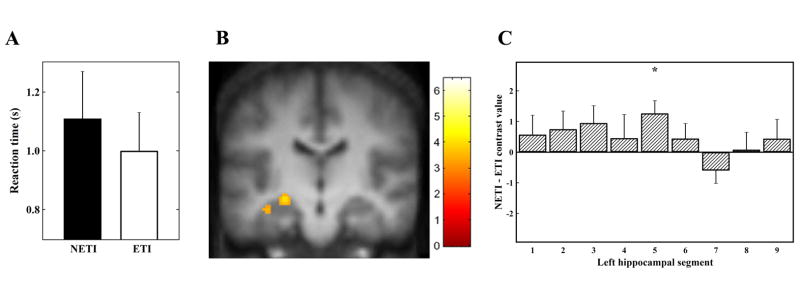
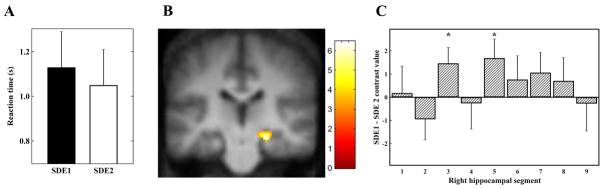
Subjects responded significantly faster when identifying novel pairs that did contain end items (i.e., ETI pairs) (1.01 ± 0.13 s) compared to those that did not contain end items (i.e., NETI pairs) (1.11 ± 0.16 s) (paired sample t-test: t(12)=3.6, P=0.004) (Figure 1A). Subjects responded significantly faster when disambiguating novel pairs with a symbolic distance of two (i.e, SDE2 pairs) (1.05 ± 0.16) compared to a symbolic distance of one (i.e., SDE1 pairs) (1.13 ± 0.16) (paired sample t-test: t(12)=4.5, P=0.001) (Figure 3A).
Figure 1.
Figure 3.
In the post-test questionnaire, all of the 13 subjects demonstrated explicit awareness of the hierarchy by 1) reporting that they recognized an ordered sequence of the six items, and 2) ordering the six items correctly according to the underlying hierarchy.
3.2. FMRI Data
3.2.1. Transitive inference
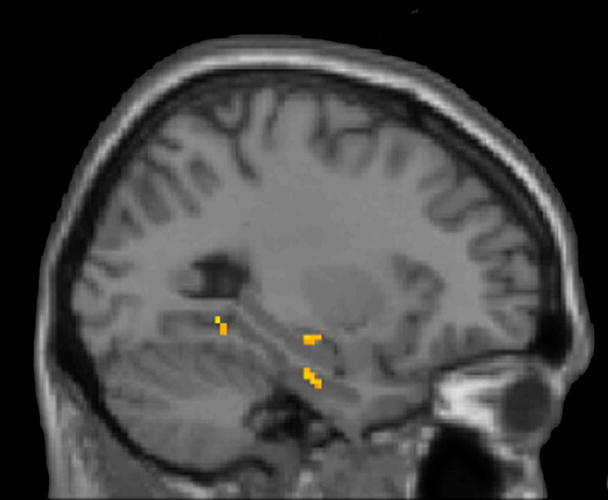
We found significantly greater left hippocampal activation (Talairach coordinates x, y, z = −24, −20, −7, Z = 3.05 and −38, −20, −16, Z = 2.75; both P<0.005) when comparing trials of NETI pairs with trials of ETI pairs (Figure 1B). This activation was readily seen in individual subjects (Figure 2). In addition, the inferior frontal gyrus, insula, precuneus, and dorsomedial thalamus were regions within the previously identified TI network that showed activation in this contrast (Table 1). There was no significant difference in other brain areas using the corrected threshold of P<0.05.
Figure 2.
Extra-hippocampal TI network activations
| Region | NETI-ETI | Z | SDE1-SDE2 | Z |
|---|---|---|---|---|
| Frontal | ||||
| L inferior frontal gyrus | −53, 18, 5 (BA 45) | 3.91 | ||
| R inferior frontal gyrus | 32, 27, −3 (BA 47) | 3.96 | ||
| 40, 30, 0 (BA 47) | 3.52 | |||
| L middle frontal gyrus | −51, 23, 28 (BA 9) | 3.29 | ||
| R middle frontal gyrus | 53, 32, 22 (BA 46) | 2.92 | ||
|
| ||||
| L Insula | −36, 20, 3 (BA 13) | 2.86 | ||
|
| ||||
| Temporal | ||||
| L Inferior temporal gyrus | −44, −49, −3 (BA 37) | 3.68 | ||
| R Middle temporal gyrus | 60, −35, 2 (BA 21) | 3.48 | ||
| L Superior temporal gyrus | −48, 13, −4 (BA 22) | 2.83 | ||
|
| ||||
| R Precuneus | 8, −40, 48 (BA 7) | 2.88 | ||
| 4, −42, 52 (BA 7) | 2.83 | |||
|
| ||||
| Parietal | ||||
| L Inferior parietal lobule | −38, −48, 40 (BA 40) | 4.00 | ||
| −26, −60, 45 (BA 7) | 3.39 | |||
| R Superior parietal lobule | 14, −63, 55 (BA 7) | 2.98 | ||
|
| ||||
| R Angular Gyrus | 34, −65, 25 (BA 39) | 3.50 | ||
| 30, −55, 36 (BA 39) | 3.48 | |||
|
| ||||
| R Dorsomedial Thalamus | 6, −2, 4 | 3.97 | ||
Extra-hippocampal TI network activations are listed for the two contrast of interest: non-end item vs. end item TI (NETI-ETI) and non end-item TI with symbolic distance of 1 vs. 2 (SDE1-SDE2). Activations are reported in Talairach in mm, where L denotes left hemisphere and R denotes right hemisphere. Z denotes Z-scores associated with the activations. BA denotes Brodmann area. All activations are reported at statistical threshold of p<0.005.
Using the anatomically guided ROI analysis, we found one segment in the left hippocampus with significantly greater activation during the identification of NETI pairs (t(12)=3.04, one-tailed P-value = 0.005) (Figure 1C). This was the fifth segment out of nine along the long axis of the left hippocampus, corresponding approximately to the mid-point of the hippocampus in each of the thirteen subjects.
3.2.2. Symbolic distance effect (SDE)
We found one region in the right hippocampus (Talairach coordinates x, y, z = 24, −24, −9; Z = 4.16), which was significantly (P<0.005) more activated during SDE1 trials compared with SDE2 trials (Figure 3B). We also found that four cortical regions within the TI network showed activation in this contrast (Table 1). There was no significant difference in other brain areas using the corrected threshold of P<0.05.
Using the anatomically guided ROI analysis, we found two segments in the right hippocampus with significantly greater activation during trials with SDE1 pairs compared to trials with SDE2 pairs (third segment: t(12)=2.15, one-tailed P-value = 0.027 and fifth segment: t(12)=2.04, one-tailed P-value = 0.032) (Figure 3C).
The hippocampal activation patterns described above are not simply due to time on task, since regression analysis showed no correlation between activation and reaction time difference between the two conditions in the hippocampal segments across subjects. Furthermore, regression analysis revealed no correlation between the degree of signal loss and the contrast of parameter estimates in individual hippocampal segments in any of the subjects, demonstrating that differences in activation between individual hippocampal segments could not be attributed to differences in signal intensity across the segments.
4. Discussion
4.1. Hippocampal contribution to TI
The goal of this study was to examine the specific role of the hippocampus in transitive inference. In this experiment, the subjects learned the overlapping sequence A>B>C>D>E>F and were then tested on transitive inferences. We hypothesized that transitive inferences on novel pairs devoid of the end items A and F (e.g., BD and CE) would lead to greater hippocampal activity than transitive inferences on novel pairs including one of the end items (e.g., AC and CF). In addition, we hypothesized that inference on non-end-item pairs with a symbolic distance of one would lead to greater hippocampal activation than inference on non-end-item pairs with a symbolic distance of two.
Our previous study (Heckers et al., 2004) of hippocampal recruitment during TI in humans employed a study design that combined ETI and NETI pairs for analysis. We were, therefore, unable to investigate the role of the hippocampus during TI judgments that did not rely on any end item information.
In the present study, we now demonstrate a specific role of the hippocampus for transitive inference contrasting pairs devoid of end items with those including end items, using a whole-brain voxel-based analysis and an anatomically guided region-of-interest analysis limited to individual subjects’ hippocampi. Thus, we show greater activation of the hippocampus in transitive inference that cannot be guided by end items and necessitates flexible manipulation of the sequence.
Using both whole-brain and ROI analyses, we also show greater hippocampal activity when items in non-end-item transitive inference pairs are closer to each other (i.e., symbolic distance of 1 versus 2). TI judgments on items in a hierarchically organized sequence take more time when items are closer to each other, which is known as SDE (Acuna et al., 2002a). SDE in TI is analogous to the finding of increased reaction times as the difference between numbers and physical magnitudes such as length, size, and luminance, decreases in comparison tasks (Pinel et al., 2001; Fias et al., 2003; Pinel et al., 2004; Ansari et al., 2005; Kadosh et al., 2005). Like numbers and physical dimensions (Hubbard et al., 2005), the sequences underlying TI judgments are thought to be stored in spatial representations (McGonigle and Chalmers, 1986; Davis, 1992), which explains the greater demands on relational processing as the distance betweeen non-adjacent items diminishes.
SDE thus indicates that subjects identify the correct order in novel pairs by accessing a hierarchical representation of the sequence. Here we show that higher degrees of sequence manipulation during TI are associated with greater hippocampal activation.
It has been suggested that the greater degree of hippocampal activation observed in relational versus single-item memory tasks is due to a greater load of items to be encoded or retrieved, rather than a specialized role for the hippocampus in relational memory (Squire et al., 2004). In this study, we find that hippocampal activation is greater for pairs BD and CE (i.e., one intervening item) than pair BE (i.e., two intervening items). This suggests that hippocampal activation scales with the degree of relational processing rather than the number of items to be recalled.
Previous reports have discussed anterior-posterior gradients and hemispheric asymmetry of hippocampal activation during memory retrieval (Lepage et al., 1998; Dolan and Fletcher, 1999; Schacter and Wagner, 1999; Greicius et al., 2003). Our finding of activation in the third and fifth segments out of the nine segments that each hippocampus was divided into (corresponding to the hippocampal body) are in line with previous studies of relational memory retrieval (Greicius et al., 2003; Prince et al., 2005). Specifically, the intermediate part of the hippocampus has been implicated in associative learning and retrieval (Small et al., 2001).
The hemispheric lateralization of hippocampal activation is a matter of active research (Henson, 2005). Lateralization during TI in the current study and in previous studies (Nagode and Pardo, 2002; Heckers et al., 2004; Ongur et al., 2006) is not easily interpreted and needs to be evaluated further.
Our finding contributes to the debate between the two prominent views on how transitive inferences are solved. In the relational flexibility account, the hippocampus links related memories according to their common features and this linkage results in a logical network that can support inferences between items in memory that are only indirectly related (McGonigle and Chalmers, 1986; Eichenbaum, 1992; Squire, 1992; Cohen and Eichenbaum, 1993; Dusek and Eichenbaum, 1997; Burgess et al., 2002; Eichenbaum, 2004). On the other hand, in the excitatory strength/value transfer account, each item from the sequence acquires a distinct associative strength through training. Performance on novel pairs is then guided by the absolute difference between the associative strengths of the items composing the novel pairs. In this account, the hippocampus plays only a minor role in transitive inference, limited to the early phases of learning associative strengths (von Fersen et al., 1991; Wynne, 1998; Frank et al., 2003; Van Elzakker et al., 2003; Frank et al., 2005). The imaging results presented here (i.e., greater hippocampal activity for NETI than ETI pairs and for smaller symbolic distances) support the relational flexibility account of hippocampal function.
In addition to making different predictions about hippocampal contribution to TI, the two views also predict different behavioral outcomes. According to the value transfer theory, the associative strengths of B and D are too similar in the six item sequence A>B>C>D>E>F, resulting in poor performance on the BD but not the BE pairing (Frank et al., 2003; Van Elzakker et al., 2003). In our study, however, all subjects demonstrated greater than 95% accuracy on the BD trial. The proponents of value transfer theory do acknowledge that their account might not be valid when subjects are aware of the sequence hierarchy (Frank et al., 2005). While our subjects were not explicitly informed of a hierarchical sequence to be detected, the training encouraged discovering this hierarchy. Moreover, training to ceiling level performance on premise pairs, as done in this study here, is known to promote awareness of the sequence (Ellenbogen et al., 2007). All our subjects indeed demonstrated post-experimental awareness of the hierarchy, and behaved in accordance with the relational flexibility account for TI.
It has been suggested that different neural networks support TI with and without awareness of the underlying hierarchy of stimulus pairs (Ellenbogen et al., 2007). Specifically, it has been hypothesized that “aware” subjects are more likely to demonstrate frontal activation as found in some previous studies (Waltz et al., 1999; Acuna et al., 2002b) whereas “unaware” subjects would rely more on the hippocampus (Libben and Titone, 2008). However, the results presented here indicate that both the hippocampus and frontal cortices (see below) are integral to TI performance in subjects who are aware of the hierarchy.
The hippocampus supports various forms of relational memory: episodic memory, associative memory, and recollection-based (as opposed to familiarity-based) memory (Moscovitch, 1995; Vargha-Khadem et al., 1997; Tulving and Markowitsch, 1998; Aggleton and Brown, 1999). This view is supported by reports of greater hippocampal activation for episodic compared with familiarity-based memory retrieval (Eldridge et al., 2000; Kensinger et al., 2003; Dolcos et al., 2005; Eldridge et al., 2005; Yonelinas et al., 2005), associative compared with non-associative memory retrieval (Bunge et al., 2004; Giovanello et al., 2004; Kirwan and Stark, 2004), and strong compared with weak autobiographical memory retrieval (Gilboa et al., 2004; Greenberg et al., 2005) in human neuroimaging studies. Deficits in TI performance in animals with hippocampal lesions further support the notion that the hippocampus is crucial for relational memory (Dusek and Eichenbaum, 1997; Buckmaster et al., 2004). Our results provide evidence for a specific role of the hippocampus in the retrieval of associative/relational memories.
4.2. Extra-hippocampal contributions to TI
Our study also confirms the role of a distributed neural network in TI performance (Acuna et al., 2002b; Heckers et al., 2004). Several brain regions were more active during trials using NETI pairs than during trials using ETI pairs, including the inferior frontal gyrus (BA 45 and 47), left insula, right precuneus and right dorsomedial thalamus. Except for area 45 in the inferior frontal gyrus, these areas are in close agreement with previous reports of cortical networks activated in TI (Acuna et al., 2002b; Heckers et al., 2004). Whereas area 45 is classically known as part of the Broca’s area involved in speech production, it is also activated in verbal analogy tasks compared with a semantic judgment task (Luo et al., 2003) and is known to support mental arithmetic and fact retrieval (Simon et al., 2004). Moreover, the adjacent area BA 44 was shown to be activated in complex reasoning task when a 2-relational problem was compared with a 1-relational problem (Christoff et al., 2001). The activation of BA 45 in NETI trials compared with ETI trials could, therefore, reflect the higher relational complexity and the greater need for retrieval of the sequence and for analogical reasoning. Similarly, area BA 47 and the precuneus increase their activity in proportion to the relational complexity of the Raven’s Progressive Matrices to be solved (Kroger et al., 2002). It is in this context that we interpret area BA 47 activation during NETI trials in the present and previous studies (Acuna et al., 2002b). The thalamus and the insula, on the other hand, show increased activity in proportion to uncertainty (Huettel et al., 2005). Their greater activation for NETI trials may, therefore, reflect the more ambiguous nature of these trials, given that both items in the pair have been previously rewarded, depending on the other item they had been paired with during training.
In the comparison of SDE1 and SDE2 trials, widespread cortical activation was also detected, including bilateral PFC, left inferior (IPL) and right superior parietal lobules (SPL), left angular gyrus, left and right lateral temporal lobe, and areas in the occipital lobe. Insight into the role of these cortical areas in the symbolic distance effect comes from investigations of number processing. The numerical continuum is known to have a spatial representation in humans (Hubbard et al., 2005) similar to TI (McGonigle and Chalmers, 1986; Davis, 1992). Analogously to the symbolic distance effect in TI where reaction times increase as the distance between two non-adjacent stimuli in the sequence decreases, reaction times increase in number comparisons as the difference between two numbers decreases (Pinel et al., 2001; Fias et al., 2003; Ansari et al., 2005). This effect extends to comparisons of many physical magnitudes, including length, size and luminance (Pinel et al., 2004; Kadosh et al., 2005).
This spatial-numerical interaction has been shown to be subserved by posterior parietal areas. In particular, the numerical distance effect has been related to IPL function (Ansari et al., 2005). Left IPL has also been shown to be particularly relevant for number comparisons (Gobel et al., 2004; Sandrini et al., 2004; Kadosh et al., 2005). More broadly, both IPL and SPL are recruited in quantitave comparisons in general, irrespective of the type of stimulus (Fias et al., 2003; Pinel et al., 2004). In addition, SPL also shows increased activity with relational complexity and manipulation of spatial relations (Kroger et al., 2002). IPL activation, in contrast, scales with uncertainty (Huettel et al., 2005).
We suggest that the parietal activation reported here for the symbolic distance effect is due to the greater difficulty in comparing items that are closer together on a spatial continuum that represents the sequence. Although the left IPL has previously been thought to subserve number comparisons exclusively (Sandrini et al., 2004), the present results suggest that it may also underlie comparisons within a non-numerical sequence. The angular gyrus has been shown to support mental arithmetic (Simon et al., 2004), which could be implicitly involved in the comparisons made during NETI trials. The dorsolateral PFC activation related to symbolic distance probably reflects its role in manipulating the sequence information (Acuna et al., 2002b). The cortical areas in the TI network work in synchrony with the hippocampus in order to flexibly represent the previously acquired sequence information.
In summary, our study provides a point of convergence between animal and human studies. It supports the theory of a crucial role of the hippocampus within a distributed cortico-hippocampal network underlying the flexible manipulation of previously learned relational information (Eichenbaum, 2004).
Acknowledgments
The authors would like to thank Dr. Anthony P. Weiss and Dr. Dost Ongur for invaluable discussions and Ms. Tali Ditman and Mr. Ian Greenhouse for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acuna BD, Sanes JN, Donoghue JP. Cognitive mechanisms of transitive inference. Experimental Brain Research. 2002a;146:1–10. doi: 10.1007/s00221-002-1092-y. [DOI] [PubMed] [Google Scholar]
- Acuna BD, Eliassen JC, Donoghue JP, Sanes JN. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002b;12:1312–1321. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behavioral Brain Science. 1999;22:425–489. [PubMed] [Google Scholar]
- Ansari D, Garcia N, Lucas E, Hamon K, Dhital B. Neural correlates of symbolic number processing in children and adults. Neuroreport. 2005;16:1769–1773. doi: 10.1097/01.wnr.0000183905.23396.f1. [DOI] [PubMed] [Google Scholar]
- Blair J, Spreen O. Predicting premorbid IQ: a revision of the national adult reading test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- Bond AB, Kamil AC, Balda RP. Social complexity and transitive inference in corvids. Animal Behaviour. 2003;65:479–487. [Google Scholar]
- Buckmaster CA, Eichenbaum H, Amaral DG, Suzuki WA, Rapp PR. Entorhinal cortex lesions disrupt the relational organization of memory in monkeys. The Journal of Neuroscience. 2004;24:9811–9825. doi: 10.1523/JNEUROSCI.1532-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain and Cognition. 2004;56:141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Christoff K, Prabhakaran V, Dorfman J, Zhao Z, Kroger JK, Holyoak KJ, Gabrieli JD. Rostrolateral Prefrontal Cortex Involvement in Relational Integration during Reasoning. Neuroimage. 2001;14:1136–1149. doi: 10.1006/nimg.2001.0922. [DOI] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. Cambridge, MA: MIT Press; 1993. [Google Scholar]
- Colombo M, Frost N. Representation of serial order in humans: a comparison to the findings with monkeys (Cebus apella) Psychonomic Bulletin & Review. 2001;8:262–269. doi: 10.3758/bf03196160. [DOI] [PubMed] [Google Scholar]
- Davis H. Transitive inference in rats (Rattus norvegicus) Journal of Comparative Psychology. 1992;106:342–349. doi: 10.1037/0735-7036.106.4.342. [DOI] [PubMed] [Google Scholar]
- DeWitt I, Weiss AP, Deckersbach T, Kunkel L, Goff D, Heckers S. Assessing hippocampal volume in schizophrenia using a standardized MRI protocol. Biological Psychiatry. 2002;51:21S. [Google Scholar]
- Dolan RJ, Fletcher PF. Encoding and retrieval in human medial temporal lobes: an empirical investigation using functional magnetic resonance imaging (fMRI) Hippocampus. 1999;9:25–34. doi: 10.1002/(SICI)1098-1063(1999)9:1<25::AID-HIPO3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek JA, Eichenbaum H. The hippocampus and memory for orderly stimulus relations. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7109–7114. doi: 10.1073/pnas.94.13.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and declarative memory in animals. Journal of Cognitive Neuroscience. 1992;4:217–231. doi: 10.1162/jocn.1992.4.3.217. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–120. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Knowlton BJ, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: a selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Eldridge LL, Engel SA, Zeineh MM, Bookheimer SY, Knowlton aBJ. A dissociation of encoding and retrieval processes in the human hippocampus. The Journal of Neuroscience. 2005;25:3280–3286. doi: 10.1523/JNEUROSCI.3420-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbogen JM, Hu PT, Payne JD, Titone D, Walker MP. Human relational memory requires time and sleep. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:7723–7728. doi: 10.1073/pnas.0700094104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fias W, Lammertyn J, Reynvoet B, Dupont P, Orban GA. Parietal Representation of Symbolic and Nonsymbolic Magnitude. Journal of Cognitive Neuroscience. 2003;15:47–56. doi: 10.1162/089892903321107819. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, O’Reilly RC. Transitivity, flexibility, conjunctive representations, and the hippocampus. II. A computational analysis. Hippocampus. 2003;13:299–312. doi: 10.1002/hipo.10084. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, Levy WB, O’Reilly RC. When logic fails: implicit transitive inference in humans. Memory & Cognition; In press. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Rudy JW, Levy WB, O’Reilly RC. When logic fails: Implicit transitive inference in humans. Memory & Cognition. 2005;33:742–750. doi: 10.3758/bf03195340. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovitch M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Giovanello KS, Schnyer DM, Verfallie M. A critical role for the anterior hippocampus in relational memory: evidence from an fMRI study comparing associative and item recognition. Hippocampus. 2004;14:5–8. doi: 10.1002/hipo.10182. [DOI] [PubMed] [Google Scholar]
- Gobel SM, Johansen-Berg H, Behrens T, Rushworth MF. Response-Selection-Related Parietal Activation during Number Comparison. Journal of Cognitive Neuroscience. 2004;16:1536–1551. doi: 10.1162/0898929042568442. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Co-activation of the amygdala, hippocampus and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Greene AJ, Spellman BA, Dusek JA, Eichenbaum HB, Levy WB. Relational learning with and without awareness: transitive inference using nonverbal stimuli in humans. Memory & Cognition. 2001;29:893–902. doi: 10.3758/bf03196418. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Boyett-Anderson JM, Eliez S, Schatzberg AF, Reiss AL, Menon V. Regional analysis of hippocampal activation during memory encoding and retrieval: fMRI study. Hippocampus. 2003;13:164–174. doi: 10.1002/hipo.10064. [DOI] [PubMed] [Google Scholar]
- Guillermo Paz-y-Mino C, Bond AB, Kamil AC, Balda RP. Pinyon jays use transitive inference to predict social dominance. Nature. 2004;430:778–781. doi: 10.1038/nature02723. [DOI] [PubMed] [Google Scholar]
- Heckers S, Zalesak M, Weiss AP, Ditman T, Titone D. Hippocampal activation during transitive inference in humans. Hippocampus. 2004;14:153–162. doi: 10.1002/hipo.10189. [DOI] [PubMed] [Google Scholar]
- Henson RN. A mini-review of fMRI studies of human medial temporal lobe activity associated with recognition memory. The Quarterly Journal of Experimental Psychology. 2005;58B (34):340–360. doi: 10.1080/02724990444000113. [DOI] [PubMed] [Google Scholar]
- Hubbard EM, Piazza M, Pinel P, Dehaene S. Interactions Between Number and Space in Parietal Cortex. Nature Reviews Neuroscience. 2005;6:435–448. doi: 10.1038/nrn1684. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under Uncertainty: Probabilistic Context Influences Activation of Prefrontal and Parietal Cortices. The Journal of Neuroscience. 2005;25:3304–3311. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadosh RC, Henik A, Rubinstein O, Mohr H, Dori H, van de Ven V, Zorzi M, Hendler T, Goebel R, Linden DEJ. Are numbers special? The comparison systems of the human brain investigated by fMRI. Neuropsychologia. 2005;43:1238–1248. doi: 10.1016/j.neuropsychologia.2004.12.017. [DOI] [PubMed] [Google Scholar]
- Kensinger EA, Clarke RJ, Corkin S. What neural correlates underlie successful encoding and retrieval? A functional magnetic resonance imaging study using a divided attention paradigm. The Journal of Neuroscience. 2003;23:2407–2415. doi: 10.1523/JNEUROSCI.23-06-02407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Medial temporal lobe activation during encoding and retrieval of novel face-name pairs. Hippocampus. 2004;14:919–930. doi: 10.1002/hipo.20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroger JK, Sabb FW, Fales CL, Bookheimer SY, Cohen MS, Holyoak KJ. Recruitment of Anterior Dorsolateral Prefrontal Cortex in Human Reasoning: a Parametric Study of Relational Complexity. Cerebral Cortex. 2002;12:477–485. doi: 10.1093/cercor/12.5.477. [DOI] [PubMed] [Google Scholar]
- Lepage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: the HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Libben M, Titone D. The role of awareness and working memory in human transitive inference. Behavioural Processes. 2008;77:43–54. doi: 10.1016/j.beproc.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Luo Q, Perry C, Peng D, Jin Z, Xu D, Ding G, Xu S. The neural substrate of analogical reasoning: an fMRI study. Cognitive Brain Research. 2003;17:527–534. doi: 10.1016/s0926-6410(03)00167-8. [DOI] [PubMed] [Google Scholar]
- Martin N, Alsop B. Transitive inference and awareness in humans. Behavioral Processes. 2004;67:157–165. doi: 10.1016/j.beproc.2004.03.017. [DOI] [PubMed] [Google Scholar]
- McGonigle B, Chalmers M. Representations and strategies during inference. In: McGonigle B, editor. Reasoning and discourse processes. London: Academic Press; 1986. pp. 141–164. [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M. Recovered consciousness: a hypothesis concerning modularity and episodic memory. Journal of Clinical & Experimental Neuropsychology. 1995;17:276–290. doi: 10.1080/01688639508405123. [DOI] [PubMed] [Google Scholar]
- Nagode JC, Pardo JV. Human hippocampal activation during transitive inference. Neuroreport. 2002;13:939–944. doi: 10.1097/00001756-200205240-00008. [DOI] [PubMed] [Google Scholar]
- Ongur D, Cullen TJ, Wolf DH, Rohan M, Barreira P, Zalesak M, Heckers S. The Neural Basis of Relational Memory Deficits in Schizophrenia. Archives of General Psychiatry. 2006;63:356–365. doi: 10.1001/archpsyc.63.4.356. [DOI] [PubMed] [Google Scholar]
- Piaget J. The Language and Thought of the Child. New York: Harcourt Brace; 1928. [Google Scholar]
- Pinel P, Dehaene S, Riviere D, LeBihan D. Modulation of parietal activation by semantic distance in a number comparison task. Neuroimage. 2001;14:1013–1026. doi: 10.1006/nimg.2001.0913. [DOI] [PubMed] [Google Scholar]
- Pinel P, Piazza M, Le Bihan D, Dehaene S. Distributed and Overlapping Cerebral Representations of Number, Size and Luminance during Comparative Judgments. Neuron. 2004;41:983–993. doi: 10.1016/s0896-6273(04)00107-2. [DOI] [PubMed] [Google Scholar]
- Prince SE, Daselaar SM, Cabeza R. Neural correlates of relational memory: successful encoding and retrieval of semantic and perceptual associations. The Journal of Neuroscience. 2005;25:1203–1210. doi: 10.1523/JNEUROSCI.2540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, Evans AC. Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cerebral Cortex. 2000;10:433–442. doi: 10.1093/cercor/10.4.433. [DOI] [PubMed] [Google Scholar]
- Rapp PR. Who’s the fairest of them all? Role of the human hippocampus in the relational organization of memory. Hippocampus. 2004;14:141–142. doi: 10.1002/hipo.20008. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Eichenbaum H. Learning and memory for hierarchical relationships in the monkey: effects of aging. Behavioral Neuroscience. 1996;110:887–897. doi: 10.1037//0735-7044.110.5.887. [DOI] [PubMed] [Google Scholar]
- Sandrini M, Rossini PM, Miniussi C. The differential involvement of inferior parietal lobule in number comparison: a rTMS study. Neuropsychologia. 2004;42:1902–1909. doi: 10.1016/j.neuropsychologia.2004.05.005. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wagner AD. Medial temporal lobe activations in fMRI and PET studies of episodic encoding and retrieval. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Simon O, Kherif F, Flandin G, Poline J-B, Riviere D, Mangin JF, Le Bihan D, Dehaene S. Automatized clustering and functional geometry of human parietofrontal networks for language, space and number. Neuroimage. 2004;23:1192–1202. doi: 10.1016/j.neuroimage.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of hippocampal formation. Nature Neuroscience. 2001;4:442–449. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- Squire L. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annual Review of Neuroscience. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Titone D, Ditman T, Holzman PS, Eichenbaum H, Levy DL. Transitive inference in schizophrenia: impairments in relational memory organization. Schizophrenia Research. 2004;68 (2–3):235–247. doi: 10.1016/S0920-9964(03)00152-X. [DOI] [PubMed] [Google Scholar]
- Tulving E, Markowitsch HJ. Episodic and declarative memory: role of the hippocampus. Hippocampus. 1998;8:198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Van Elzakker M, O’Reilly RC, Rudy JW. Transitivity, flexibility, conjunctive representations, and the hippocampus. I. An empirical analysis. Hippocampus. 2003;13:334–340. doi: 10.1002/hipo.10083. [DOI] [PubMed] [Google Scholar]
- Vargha-Khadem F, Gadian DG, Watkins KE, Connelly A, Van Paesschen W, Mishkin M. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277:376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- von Fersen L, Wynne CDL, Delius JD, Staddon JER. Transitive inference formation in pigeons. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:334–341. [Google Scholar]
- Waltz JA, Knowlton BJ, Holyoak KJ, Boone KB, Mishkin FS, de Menezes Santos M, Thomas CR, Miller BL. A system for relational reasoning in human prefrontal cortex. Psychological Science. 1999;10:119–125. [Google Scholar]
- Wynne CDL. A minimal model of transitive inference. In: Staddon JER, editor. Models of action. Mahwah, N.J: Lawrence Erlbaum Associates; 1998. pp. 269–307. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. The Journal of Neuroscience. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]