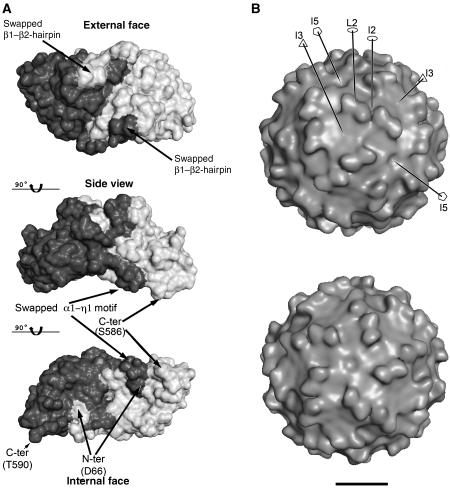
Figure 2.
Surface features of the PBV capsid. (A) CP dimer with the subunits in different shades of grey. Top and bottom are views down the L2 axis, from outside and inside the VLP, respectively, with a side view in the middle. The internal curvature of the CP dimer matches roughly the internal radius of the particle. Note the intricate interface between subunits, generated by the N-terminal β1β2 and α1-η1 swapping, as indicated. The location of the N-terminus marks the site of the transproteolytic cleavage. (B) Surface of the VLP. cEM 3D-reconstruction showing the presence of 60 dimeric protrusions formed by domain P. The contour level was chosen to accommodate the volume of the CP dimer in the icosahedral asymmetric unit. A few symmetry axes are labelled for orientation. Top and bottom panels are seen down the I3 and I5 axes, respectively, slightly miss-oriented in order to help visualize certain features, like the cleft at the I2 axes, the I5 depression and the flat I3 region. Some of the symmetry axes are drawn, with standard symbols for 2-, 3- and 5-fod axes (empty ellipse, triangle and pentagon, respectively) and labelled. Bar: 100 Å.