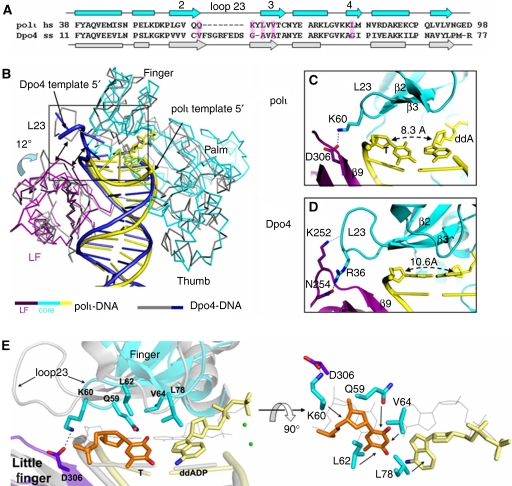
Figure 3.
Polι and Dpo4 active site comparison. (A) Structure-based sequence alignment of amino acids for the finger domains of polι (cyan) and Dpo4 (grey). Numbres 2, 3 and 4 indicate the second, third and fourth β-sheets. Secondary structure is indicated as rectangles for α-helices and arrows for β-sheets. Residues interacting with the replicating base pair are highlighted in magenta. (B) Superposition of T:ddADP (cyan, purple) and ternary Dpo4 (type I)–DNA–nucleotide (1JX4, grey). The incoming nucleotides are shown as sticks for Dpo4 (grey) and T:ddADP (yellow). LF represents the little finger domain. (C, D) Close-up views of active sites showing finger–LF domain interactions in polι and Dpo4. Finger domains are cyan, LF domains are purple and DNA is yellow. (E) Active site superposition from (B) of T:ddADP (finger: cyan; little finger: purple; T base: orange; ddADP: yellow) with Dpo4 (grey). Positioning of the replicating base pair by polι side chains is indicated with black arrows.