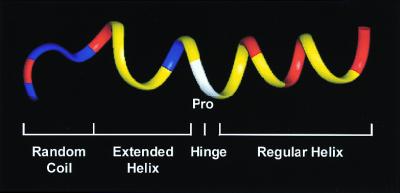
Figure 1.
Ribbon-model representation of the backbone structure of buforin II in 50% trifluoroethanol. The N-terminal random coil, the extended helix, the hinge, and the C-terminal regular helix form an overall amphipathic structure. The amino acid residues are colored as follows: positively charged residues, red; other hydrophilic residues, blue; proline, white; other hydrophobic residues, yellow.