Abstract
Purpose
Age-related changes in tongue function may contribute to dysphagia in elderly people. Our purpose was to investigate whether aged rats that have undergone tongue exercise would manifest increased protrusive tongue forces and increased genioglossus (GG) muscle fiber cross sectional areas.
Method
Forty-eight young adult, middle-aged and old Fischer 344/Brown Norway rats received 8 weeks of tongue exercise. Protrusive tongue forces were measured before and after exercise. GG muscle fiber cross sectional area was measured in exercised rats and compared with cross sectional areas in a no-exercise control group.
Results
A significant increase in maximum tongue force was found following exercise in all age groups. In addition, a trend for increased GG muscle fiber cross sectional area, and a significant increase in variability of GG muscle fiber cross sectional area were identified post-exercise.
Conclusion
The findings of this study have implications for treatment of elderly persons with dysphagia using tongue exercise programs. Specifically, increases in tongue force that occur following 8 weeks of progressive resistance tongue exercise may be accompanied by alterations in tongue muscle fiber morphology. These changes may provide greater strength and endurance for goal-oriented actions associated with the oropharyngeal swallow and should be investigated in future research.
Keywords: tongue, aging, rat, tongue exercise, genioglossus, swallowing, deglutition
Introduction
Sarcopenia, the age-related loss of skeletal muscle mass, strength, and function (Rosenberg, 1997) is caused by multiple factors (Doherty, 2003). A major component of sarcopenia is muscle atrophy, which is characterized by a reduction in muscle fiber cross sectional area. Muscle size appears to be related to changes in neuromuscular function across the lifespan, with age-related atrophic changes contributing to strength reductions (Vandervoort, 2002).
Importantly, muscle size and function can be modified via physical activity and exercise, such that increased activity is associated with a slower rate of age-related motor decline (Buchman et al., 2007). With progressive resistance training, elderly men and women have increased limb muscle size and strength, even those who were 90 years old or older (Frontera et al., 1988; Fiatarone et al., 1990; Grimby et al., 1992; Tracy et al., 1999; Vincent et al., 2002).
Progressive resistance training programs are generally focused on increasing muscle strength via high load resistance tasks, such as weight lifting, with loads that approach the maximum capacity of an individual muscle group. Specific criteria are used to determine the maximum capacity for a particular individual and muscle group, and are determined behaviorally. That is, individuals are asked to lift a weight near their suspected maximum, and the maximal weight that can be moved through the full range of motion without extraneous body motion is termed the “one-repetition maximum” (1-RM) (Taaffe et al., 1999). Exercise programs are then planned over a course of weeks, in which approximately 10 repetitions of the resistance task are performed at progressively increasing loads relative to the 1-RM (i.e. 40% to 60% of the 1-RM) on 3 nonconsecutive days per week (Taaffe et al., 1999; Vincent et al., 2002). While these high intensity resistance tasks, with relatively low numbers of repetitions, are generally recommended for younger adults, gains in strength can also be achieved in elderly research participants with lower intensity tasks involving high numbers of repetitions (Taaffe et al., 1996; Vincent et al., 2002). In addition, 1 day per week of resistance exercise, or performance of only one set of 10 repetitions, has resulted in significantly improved muscle strength in elderly subjects, and also in improving temporal features of a functional motor action (i.e. rising from a chair) (Taaffe et al., 1999; Galvao and Taaffe, 2005). Accordingly, the parameters of effective exercise programs for elderly persons may be quite different from those prescribed for young adults (Vincent et al., 2002).
Functional gains in endurance have also been observed following resistance training, and these improvements were progressive and generalized to improved functional mobility in frail elderly persons (i.e. stair climbing times) (Fiatarone et al., 1990; Vincent et al., 2002). The mechanisms of these improvements were presumed to be hypertrophy of the muscle, neuromuscular adaptation, or a combination of the two influences (Frontera et al., 1988; Grimby et al., 1992; Tracy et al., 1999). Therefore, resistance exercise programs have resulted in improved muscle strength in elderly people, and have translated into improved endurance, functional mobility (Fiatarone et al., 1990), and performance of functional goal-oriented motor tasks (Taaffe et al., 1999; Vincent et al., 2002).
It is very likely that sarcopenia in cranial muscles contributes to age-related decline in the critical functions of the head and neck, including swallowing ability (Baum and Bodner, 1983; Ekberg and Feinberg, 1991; Robbins et al., 1992; McKee et al., 1998; Logemann et al., 2000). Parameters of swallowing function change with aging such that older individuals swallow more slowly, resulting in an increased opportunity for airway penetration and aspiration (Robbins et al., 1995). Lingual dysfunction may contribute to age-related swallowing problems, and prior work has discovered reduced or otherwise altered lingual pressures during maximum function tasks in elderly people (Mortimore et al., 1999; Nicosia et al., 2000). While the direct mechanisms for tongue force reductions are unclear, they may be due to changes in tongue muscle morphology and strength with aging. Data concerning patients with stroke, head and neck cancer, amyotrophic lateral sclerosis, and other conditions have shown a relationship between tongue weakness, increased oral transport time for a bolus during swallowing, and impaired swallowing function. Reductions in tongue muscle fiber diameter have been reported with increasing age for the superior longitudinal muscle in human cadaveric tongues, where reductions in fiber diameter began at age 40 in males and age 30 in females (Nakayama, 1991). Accordingly, muscles of the tongue that contribute to the oropharyngeal swallow may be particularly affected by aging and tongue weakness has been linked with swallowing impairment in patients with dysphagia (Lazarus et al., 1996, 2000, 2003; Mortimore et al., 1999; Nicosia et al., 2000; Clark et al., 2003; Robbins et al., 2007).
Rehabilitation of the aging swallow is aimed at preventing or reversing pathological aspects of the swallow via appropriate compensatory methods or direct treatment of the pathology. If tongue weakness is contributing to poor swallowing outcomes in older people, then exercise of this end organ may prove beneficial, and tongue exercise has been proposed and used as a treatment for dysphagia (Robbins et al., 2005, 2007, 2008; Kays and Robbins, 2006; cf. Stathopoulos and Duchan, 2006). Data from research studies suggest that different types of exercises may be effective in treating the swallowing disorders of people with compromised health (Shaker et al., 1997; El Sharkawi et al., 2002; Robbins et al., 2005, 2007; Kays and Robbins, 2006). In particular, 8 weeks of progressive resistance exercise of the tongue using the Iowa Oral Performance Instrument (IOPI) in 10 post-stroke research participants resulted in increased maximum isometric tongue pressures, increased tongue pressures during swallowing, improved temporal measures of swallowing, and improved swallowing-related quality of life (Robbins et al., 2007). Two of the three participants studied via magnetic resonance imaging of the tongue also demonstrated increased tongue volumes following the exercise program (Robbins et al., 2007). As such, increased tongue muscle activation and/or strength and effort training may increase tongue muscle function, and in particular, tongue muscle function in relation to swallowing actions. However, investigation of the underlying biological mechansms of these changes in tongue muscle function and structure are difficult to perform in living human subjects.
To investigate this issue, we developed a model of tongue exercise in young adult, middle-aged, and old rats. Our purpose was to develop an animal model for investigations related to the use of tongue exercise as a therapeutic intervention, to investigate whether adult rats in all age groups would produce increased levels of protrusive tongue force following 8 weeks of progressive resistance exercise, and to investigate if tongue muscle fiber cross sectional area would increase with exercise. The genioglossus (GG) muscle was selected for study because the protrusive force task involved in the tongue exercises likely involved primary GG involvement. The hypothesis of this study was that young adult, middle-aged, and old rats that have undergone a program of tongue exercise with progressive resistance would manifest increased protrusive tongue forces and increased GG muscle fiber cross sectional areas relative to rats in a no-exercise condition. Tongue protrusion forces were examined because they are teleologically relevant to food intake actions in the rat and are related to the tongue exercise task employed in this study and in human clinical uses of tongue exercise.
Methods and Materials
This study was performed in accordance with the PHS policy on care and use of laboratory animals, the NIH guide for care and use of laboratory animals, and the animal welfare act. The animal use protocol was approved by the institutional animal care and use committee (IACUC) of the University of Wisconsin School of Medicine and Public Health.
Animals
Forty-eight male Fischer 344/Brown Norway rats were studied across 13 weeks in 4 separate study cohorts. The Fischer 344/Brown Norway is an inbred strain with a median lifespan of approximately 33 months for males (Turturro et al., 1999). Nine to 18 rats were studied per cohort and each contained animals that were 6 months old (young adult), 21 months old (middle aged), and 29 months old (old) when the study began. All rats were obtained from a National Institutes of Health NIA (National Institute on Aging) Aged Rodent Colony approximately 4 weeks prior to the start of the tongue exericse program to allow acclimation to the animal care facility, reversal of light cycle, water restriction, and familiarization to the operandum. These aspects of the study protocol are all described below.
At study completion, the animals were in the following age groups: (1) Young Adult: Sixteen 9-month-old rats were used with 8 animals in exercise and 8 animals in no-exercise conditions; (2) Middle Aged: Sixteen 24-month-old rats represented a time point consistent with the onset of significant aging effects in tongue, sternomastoid, thyroarytenoid, and muscles of the rat hindlimb (Rosenheimer, 1990; Balice-Gordon, 1997; Connor et al., 2002) with 8 animals in exercise and 8 animals in no-exercise conditions; and (3) Old: Sixteen 32-month-old rats allowed investigation of age-and exercise-related changes at a more advanced stage of senescence. Within the old group, 7 exercise rats and 7 no-exercise rats were included in the analysis; 2 old rats expired prior to study completion.
Animal preparation and training are summarized in Table 1. Following the 4 week acclimation period, half of the rats in each age group were randomly assigned to an exercise group or a no-exercise control group. While all animals were light-cycle reversed and water-restricted, only the exercise group animals received increment testing and tongue exercise with the operandum, as discussed below. As such, tongue force measures were available only for the animals within the exercise group and within-subjects comparisons were performed between baseline and post-exercise tongue forces. It was not possible to obtain voluntary tongue forces in the no-exercise group without training animals to perform the tongue force task, which would necessarily involve 6–10 days of tongue exercise at baseline and at 8 weeks following baseline. Accordingly, no-exercise control animals were used only for between-subjects analyses of tongue muscle morphometry, and not tongue force analyses.
Table 1.
Summary of the stages of animal preparation, measurement and training following arrival and acclimation to the animal care facility. EMP= estimated maximum press; 99th percentile of observed maximum force distribution. MVTF = maximum voluntary tongue force; SD= standard deviation
| Stages of Training | |||
|---|---|---|---|
| Days | Stage | Description | Measures |
| 1–14 | Animal preparation | Arrival and acclimation to animal care facility, light cycle reversal, and Water restriction to 3 hr/day | None |
| 15–25 | Introductory Period: Force Operandum Acclimation | Trained to operate force operandum | Number of tongue presses > 0.2 g |
| 26–28 | Baseline Increment Testing | Force threshold incremented in 0.2g steps |
|
| 29–84 | Tongue Force Exercise | Trained at 50, 60, 70, 80% of EMP |
|
| 85–87 | Post-exercise Increment Testing | Force threshold incremented in 0.2g steps |
|
Light Cycle Reversal
Light cycle reversal ensured that exercise was provided at the time of most activity for the rats. Upon arrival at the animal care facility all of the animals were subjected to a light cycle reversal protocol to change the light cycle by 2 hours everyday until it reached the desired time frame of 8 PM – 8 AM. Complete light cycle reversal took 7 days to complete, and the animals remained on the reversed cycle for the duration of the experiment. All experimentation and training was performed in the dark, with partial red illumination.
Water Restriction
All animals were placed on a water restriction protocol, including control animals. For exercise group animals, water restriction was instituted to establish a water reward system for achievement of tongue force goals in the learning paradigm. Following light cycle adaptation, water bottles were removed from all housing cages and animals were put on a 5-day progressive water restriction paradigm that required water access from a secured reservoir placed within a rat enclosure designed to fit the rat head (Figure 1). The progressive water restriction program restricted water access to 8 hours on Day 1, with a gradual reduction to 3 hours on Day 5. Three hours of water exposure was determined by our IACUC to be the least restrictive program for rats that allowed water to be useful as a reinforcer in our behavioral studies, but did not present a substantial compromise to animal health or well being (Toth and Gardiner, 2000). Thus, all animals were fully water restricted when receiving 3 hours of water per day and remained water restricted for the duration of the experiment.
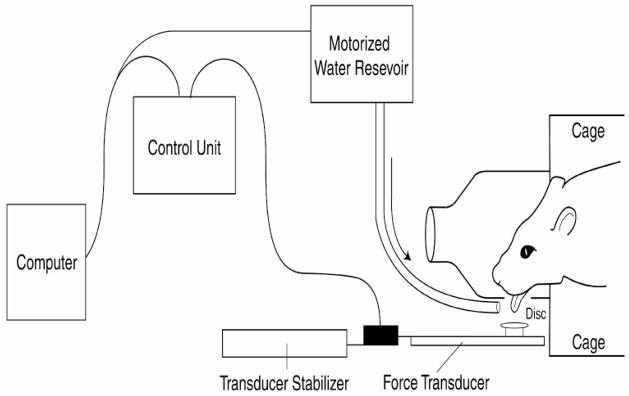
Figure 1.
Schematic of the tongue force operandum.
During tongue force training with the operandum within the exercise group, our protocol was to present water to the animals during the 10-minute exercise period as a reward when tongue force challenges were met, and then to provide unlimited water for 2 hr 50 min following exercise, with a 30-min interval of no water exposure immediately following exercise to discourage an association between removal from the operandum enclosure and transfer to a cage with unlimited water. Control rats received water for only 3 hours per day 8 weeks without any tongue exercise programming. All animals received unlimited food during the 3 hours of water exposure. Food was also available ad libitum to animals in their home cages in the animal care facility, but without water. Animals were weighed daily during the 8-week study period.
Operandum
Lick frequency, force, and duration have been successfully quantified in rodent models using operant techniques across several published studies over the last decade (Fowler and Mortell, 1992; Moss et al., 2001, 2002; Stanford et al., 2003; Smittkamp et al., 2008). We used these proven techniques as a model for the development of a custom instrument for modifying and acquiring tongue forces in rats (Figure 1).
The animal enclosure was designed to accommodate a single rat, and had a depressed front panel within a cone-shaped head enclosure and a 1 × 1 cm portal that allowed tongue access to the force operandum disk, with water presentation across the disk region. The disk was housed within an apparatus that functioned under computer control to measure parameters of the tongue press and to deliver a water reward if preset force challenges were met. The shape of the chamber and the portal access generally prevented the use of the animal’s nose, teeth, or feet to bypass the tongue press required for receipt of the water reward. However, animals had to be observed throughout the exercise training sessions to record instances in which animals used a body part other than the tongue to press the disk. All such occurrences were noted and removed from tongue force analyses. Output of the force transducer was sampled at 40 Hz with a precision of 0.2 g using custom designed computer data acquisition software (Matrix Product Development, Cottage Grove, WI). This sampling rate was adequate for the 6 Hz tongue press movements recorded and did not interfere with delivery of the water reward. Parameters sampled by the device included number of tongue presses per session and the peak force of each tongue press.
Learning Paradigm
Use of the operandum involved a traditional learning paradigm in which rats in the exercise group were trained to push the disk with the tongue. In preparation for the tongue exercise period of 8 weeks, an introductory period of 6–10 days was used for acclimation to the force operandum. For the first 3–5 days of the introductory period, water was manually dispensed when animals approached the force acquisition disk. The last 3–5 days of the introductory period required a tongue press of at least 0.2 g for obtaining an automatically-dispensed water reward. Exact number of days for the introductory period varied depending upon each animal’s performance and learning. For the tongue exercise days that followed, water was dispensed for successful tongue presses at or above a preset force threshold. The threshold was set individually for each rat based on the results of increment testing, described below.
Increment Testing
In the exercise group, an increment testing protocol was used to obtain an estimate of each rat’s maximum tongue force capacity, which we called the “estimated maximum press (EMP).” The EMP was a measure used solely to establish force thresholds for use during the ensuing 8 weeks of exercise.
Increment testing involved progressively increasing force targets during sessions on 3 consecutive days. By rapidly increasing the force increments required for obtaining a reward, the animal generally responded by increasing the force of the tongue press until a maximum force value was obtained. Data obtained through increment testing were used to estimate upper percentiles of tongue force for each animal. A von Mises-Jenkinson generalized extreme value distribution (GEV) (Coles, 2001) was fitted via maximum likelihood and its 99th percentile was obtained. The GEV fits were obtained by combining the data from all 3 days of increment testing. The EMP value for each rat was generally a value larger than the tongue forces actually observed for that animal and was used only to establish force thresholds (50%, 60%, 70%, and 80% of the EMP) for delivery of a water reward during exercise sessions.
In addition to the EMP value, increment testing was also used to record the actual, observed maximum tongue press values and number of tongue presses prior to the tongue exercise program (baseline increment testing) and following the 8-week tongue exercise period (post-exercise increment testing). The average of the largest 10 force values obtained and recorded over the 3 days prior to and following training was defined as the rat’s maximum voluntary tongue force (MVTF). We also recorded the number of tongue presses obtained during the first 2 minutes of increment testing, prior to and following the 8-week tongue exercise program.
Tongue Exercise Program
The tongue exercise program began immediately following the baseline increment testing days. The exercise period lasted 8 weeks with exercise performed 5 days per week. Eight weeks of exercise was based upon preliminary data in our laboratory suggesting that voluntary tongue force maxima were not obtained within a shorter time period. Our preliminary experiments also indicated that exercise sessions 5 days per week were necessary to maintain animal performance levels. That is, fewer sessions per week were associated with reductions in force levels following a “day off” and reduced levels of participation in the tongue force task.
Each exercise period was 10 minutes in duration, to capture the time period of most activity and participation. Tongue press forces were automatically recorded. Within an exercise session, animals produced at least 30 tongue presses greater than or equal to the target threshold for that session and received a water reward for forces greater than or equal to target values. For Weeks 1 and 2, animals were required to press the operandum with their tongue at 50% of the EMP value to obtain a reward using a variable ratio (VR) 5 reinforcement schedule. That is, on the average, rats received a water reward for every 5 responses at or above the threshold value, with some variance around the 5 response criterion. Force targets were incremented biweekly during the treatment period based on the EMP. As such, force requirements were raised to 60% for the second exercise period (Weeks 3 and 4), and increased again to 70% of the EMP for the third exercise period (Weeks 5 and 6). For the final two-week period (Weeks 7 and 8), the threshold criterion for a water reward was increased to 80% of the EMP. Values for percent of maximum force and number of resistance trials within a session are consistent with recommendations for strength training for humans by the American College of Sports Medicine (Kraemer et al., 2002).
The force of each tongue press was recorded for all exercise days and an average force per day was calculated. In addition, the number and the rate of tongue presses was recorded for each training day. At the completion of the 8-week training period, post-exercise MVTF was recorded in a manner identical to the increment testing performed for determination of the animal’s baseline MVTF. That is, an animal was placed indvidually into the operandum enclosure and force targets were progressively increased to elicit progressively increasing tongue force output during sessions on 3 consecutive days. The 10 greatest force values obtained over the 3 days were averaged. The average was defined as the animal’s post-exercise MVTF. Following the 3-day increment testing period, animals were euthanized, tongues were extracted, and muscles were dissected for determination of muscle fiber cross-sectional area.
Muscle Morphometry
Control and exercise group rats were anesthetized with isoflurane, followed by sodium pentobarbital (50 mg in 1 mL of water via intraperitoneal [IP] injection). Anesthetized animals were euthanized with Beuthanasia via cardiac injection (0.2 mL). Immediately following dissection from the host animal, genioglossus (GG) muscle specimens were individually frozen in 2-methylbutane cooled by liquid nitrogen and stored in a −80°C freezer until further processing. The GG was chosen for study because it is a large, easily identified muscle that acts to protrude the tongue, which is consistent with the action required in the tongue exercise program.
Serial 8 μm cross-sections were made from the midsection of the GG muscle using a −20 °C cryostat (Leica CM 1850). Every third section was collected and 3 sections were mounted per slide. Sections were air-dried for 45 minutes prior to staining. The immunostaining protocol involved washing the slides in phosphate buffered saline (PBS, Sigma, St. Louis, MO), fixing in 4% paraformaldehyde (Sigma, St. Louis, MO), washing again, and blocking for 2 hours in 5% goat serum (Invitrogen, Carlsbad, CA), then washing again. Slides were incubated with diluted primary antibody (polyclonal rabbit anti-laminin, Sigma, St. Louis, MO). Following PBS wash, slides were incubated with the fluorescent secondary antibody (Cy3 conjugated goat anti-rabbit IgG, Jackson ImmunoReseach, West Grove, PA) and wrapped in foil to preserve fluorescence at room temperature. Negative control slides were stained following the same procedure with an exception of omission of the primary antibody. Slides were kept at 4 °C to preserve fluorescence until microscopy was performed.
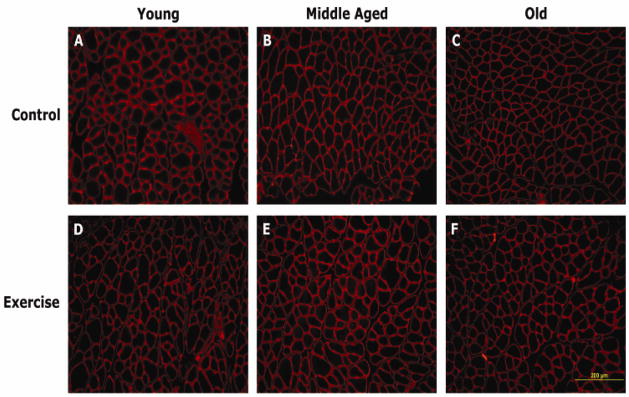
Microscopists (JAR or LM) were masked to age or exercise group. Photographs of the images were made with a Nikon Eclipse E600 microscope (Nikon, Melville, NY) and an Olympus DP70 Microscope Digital camera (Olympus, Orangeburg, NY). Fluorescence observation was made using the mercury lamp as a light source, with filtered excitation wavelength of 546/10, which emitted a deep red fluorescence when excited by green-yellow light. Images were captured at 40X magnification. Image J (NIH, Bethesda, MD) was used for image analysis to obtain between 150–230 muscle fiber cross-sectional areas per muscle sample. At least 3 different, non-overlapping regions within the sample were used to determine average size and variability of fiber areas. Representative images from a young adult, middle-aged, and old animal are shown in Figure 2.
Figure 2.
Representative cross sections of the genioglossus (GG) muscle in young adult, middle-aged and old rats in the control (no-exercise) and exercise groups.
Measures of GG muscle fiber cross-sectional area (μm2) were obtained with computer-assisted image analysis of immunostained muscle cross-sections. Muscle fiber regions were automatically selected and measured by the software. The muscle fiber areas per animal, across the 3 GG muscle regions, were sorted from largest to smallest and the 100 largest fibers per animal were used in statistical analyses. Selection of the largest muscle fibers per animal removed the possibility of including small, non-muscle connective tissue bodies or regions in the measurements.
Statistical Analyses
Within the exercise group, paired t-tests were used to compare average MVTF (g), percent change in MVTF, and number of tongue presses per 2 minutes of increment testing prior to and following tongue exercise. Two-way analysis of variance (ANOVA) examined age effects, treatment (exercise) effects, and age by exercise interactions to determine if muscle fiber cross sectional area (μm2), variability in muscle fiber cross sectional area (μm2), and body weight were significantly different as a function of age or exercise. All analyses were performed with SAS statistical software (SAS Institute Inc., Cary, NC). The critical value for obtaining statistical significance was set at α=.05 level.
Results
Tongue Exercise Program
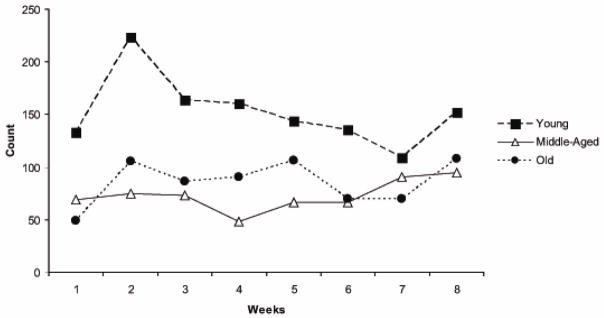
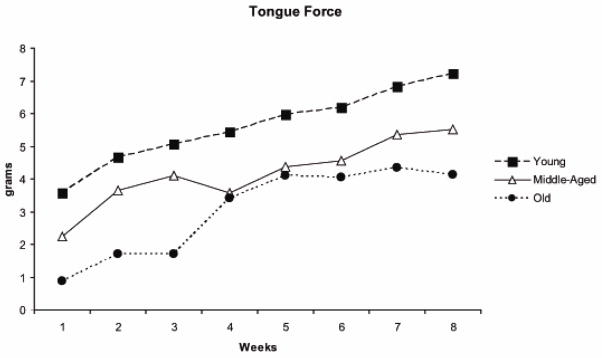
Rats in all 3 age groups participated fully in the voluntary tongue exercise program across the 8 weeks by pressing the tongue against the force operandum at least 30 times within a session at or above the pre-set threshold value. On the average across the 8 weeks, the rats in the 3 age groups produced approximately 100 tongue presses per session at or above threshold (Young Adult: 102; Middle-aged: 93; Old: 108 tongue presses per session). The average number of tongue presses at or above threshold per session for each week of exercise is shown in Figure 3 for a representative animal in each of the 3 age groups. Corresponding maximum tongue forces (averages of the 10 largest tongue press values within one session for each week) are shown for the same animals in Figure 4. It can be appreciated that there was a progressive increase in tongue force for these representative young adult, middle-aged, and old animals. The young adult animal demonstrated the greatest tongue force values and tongue press numbers over all, and the old animal the smallest force values.
Figure 3.
The number of tongue presses at or above a preset threshold level in one representative rat per age group across the 8 weeks of tongue exercise training.
Figure 4.
Maximum tongue force averages (i.e. average of the top 10 forces produced in one session per week) in one representative rat per age group across the 8 weeks of tongue exercise training.
Maximum Voluntary Tongue Force
When baseline (pre-exercise) and post-exercise increment testing data were examined across age groups, a significant increase in MVTF was observed using paired t-tests (t [22]=11.44, p<.0001; Figure 5). There was an average increase in tongue force of 222.9% over the 8-week exercise period, with the largest gains observed in the young group (mean = 254.2%) in comparison with middle-aged (248.6%) and old (158%) animals. Paired t-tests within each age group also revealed statistically significant force increases following exercise within young adult, middle-aged, and old animal groups (t > 7.7; p < .01; df = 7, young adult, middle-aged, and 6 old). When paired comparisons of the age groups were performed, a significantly greater increase in MVTF from baseline was found in the young adult versus the old animals (t [14]=2.75; p=.02), while young adult versus middle-aged and middle-aged versus old were not found to be significantly different from each other.
Figure 5.
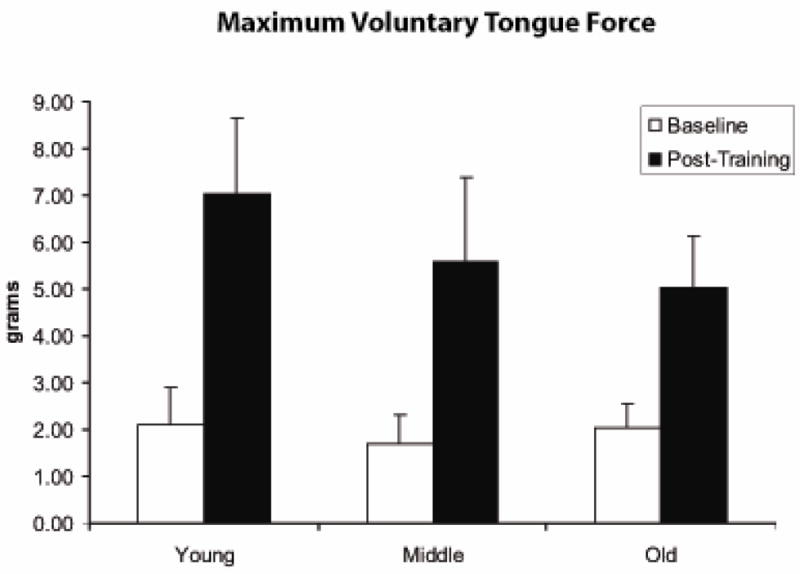
Maximum voluntary tongue force (MVTF; g) was significantly greater following 8 weeks of tongue exercise (post-training) versus baseline measures within the exercise group. (t=11.44; p<.0001).
In Figure 5, it can also be appreciated that there was not a significant difference in MVTF values across age groups at baseline (F=0.82, p=.46). However, following the 8-week exercise period, MVTF was significantly greater in the young rats versus the old rats on a post hoc LSD test (F [2,20]=3.35, p=.055; LSD p=.02). The significant difference between old and young in the 8-week data may be due the greater tongue force gains associated with exercise in the young animals versus the old animals (t [14] =2.75; p= .02). Tongue force (MVTF) following training was significantly correlated with initial, baseline levels of tongue force (r=.69; p=.0002), suggesting that greater initial force levels were associated with higher post-training forces.
The number of tongue presses within the first two minutes of increment testing (Figure 6) increased significantly following the 8-week tongue exercise period across all age groups on paired t-tests (t [23] =4.13, p=.0004).
Figure 6.
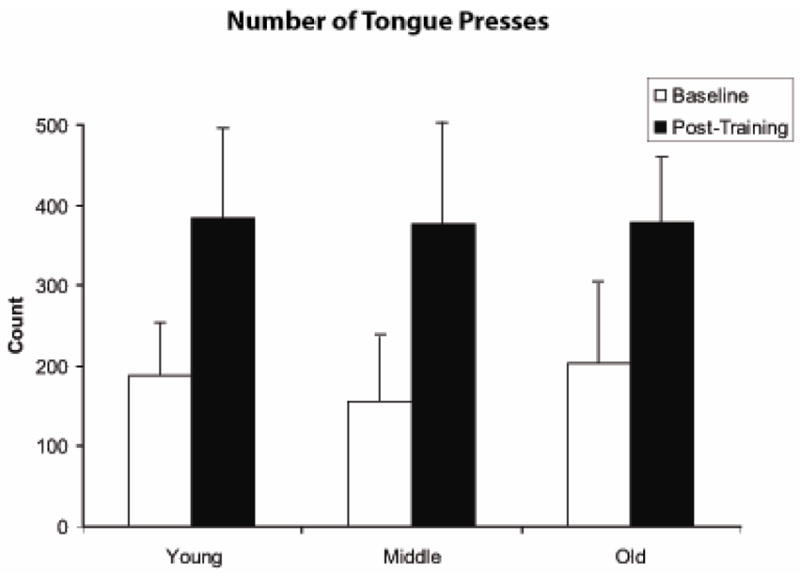
Number of tongue presses per two minutes significantly increased following 8 weeks of tongue exercise across all age groups (t=4.13; p=.0004).
Muscle Fiber Cross Sectional Area
Pre-exercise muscle fiber cross sectional area (μm2), as shown in Figure 7, was not significantly different as a function of age (F [2,40] =.95, p=.39), but there was a trend toward larger cross sectional area following the 8-week exercise period in comparison with control animals (F [1,40] =3.17, p=.08). There was not a significant age by exercise interaction effect (F [2,40] =.21, p=.80). Variation in muscle fiber cross sectional area (μm2) was examined by averaging the standard deviations in muscle fiber cross sectional area of the 100 muscle fibers with the largest areas within an animal and is shown in Figure 8. No age effect was observed (F [2,40]= .66; p=.52), but a significant difference between the exercise and control groups was observed such that variation was significantly greater in the animals that received 8 weeks of tongue exercise (F [1,40] =4.83; p=.04). No interaction between age and exercise was observed (F [2,40] =.62; p=.54).
Figure 7.
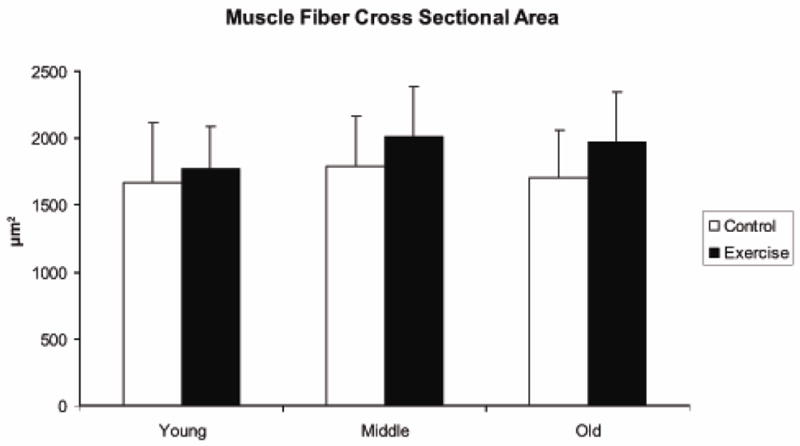
Muscle fiber cross sectional area (μm2) was not significantly different as a function of age (F=.95,p=.39), but there was a trend toward larger cross sectional area following the 8-week exercise period in comparison with control animals (F=3.17, p=.08)
Figure 8.
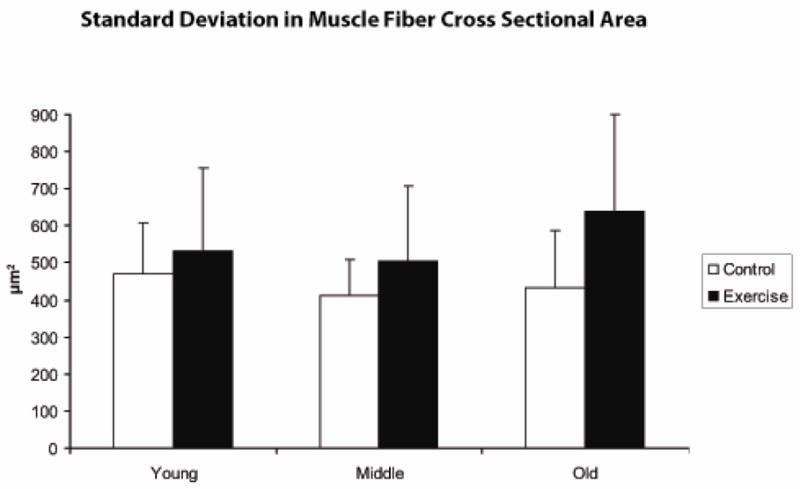
Variation (in mean standard deviation [SD]; μm2) was significantly larger in the exercise group in comparison with control animals.
Prior to and following the 8-week research period, animals randomly assigned to the tongue exercise group had a significantly smaller body weight (g) than the control animals (Table 2; F [1,40]=5.43, p=.03; F [1,40] =6.73, p=.01, respectively). While middle-aged and old animals had significantly greater body weight than young adult animals prior to and following the 8-week research period (F [2,40]=109.25, p<.0001; F [2,40] =42.14, p<.0001, respectively), there was not a significant age by exercise interaction (F [2,40]=.94, p=.40; F [2, 40]=.45, p=.64, respectively). When weight change data from baseline to 8-week assessment were examined, there was a significant main effect for age [F [2, 40] = 33.7, p < .0001]; the old animals lost significantly more weight (average loss of 50 g) than the middle-aged (average loss of 6.75 g; p <.0001) or young adult animals (average gain of 22.6 g; p <.0001). Change in weight was not significantly different in the exercise versus control groups (F [1, 40]=1.53; p=.22), and there was not a significant age by exercise interaction (F [2,40] =.47, p=.63).
Table 2.
Animal weight (g) at baseline, following the 8-week research period, and post-treatment change in weight following baseline. Data are expressed as averages ± standard deviation.
| Control (no exercise) Group | Tongue Exercise Group | |||||
|---|---|---|---|---|---|---|
| Baselline | 8 weeks | Mean Change in weight | Baseline | 8 weeks | Mean Change in Weight | |
| Young | 387.7 ± 42.7 | 410.4 ± 49.1 | 22.6 | 360.5 ± 21.0 | 383.1 ± 28.4 | 22.5 |
| Middle-Aged | 547.6 ± 35.1 | 549.1 ± 49.3 | 1.5 | 541.9 ± 32.1 | 527.0 ± 27.0 | − 15.0 |
| Old | 550.2 ± 36.5 | 505.4 ± 41.8 | − 44.7 | 509.0 ± 44.3 | 454.4 ± 59.8 | − 54.6 |
Discussion
We found that protrusive tongue forces increased significantly for all age groups following 8 weeks of tongue exercise, with greater force gains in young adult animals versus middle-aged and old rats. It is notable that even the old rats had gains in voluntary tongue force, on the average, of 158% relative to baseline levels. We also found a trend toward increased, but not significantly increased, GG muscle fiber cross sectional areas in rats that received 8 weeks of exercise when compared with no-exercise control animals. This trend was observed for rats in all age groups, even in the face of weight loss. Eight weeks of tongue exercise was also associated with a significant increase in variability of muscle fiber cross sectional area in the largest GG muscle fibers measured per animal. Increased variability in muscle fiber cross sectional area suggests that 8 weeks of targeted tongue exercise may be associated with change in muscle size, but not uniformly across the muscle. Accordingly, these findings indicate that rats can be trained to voluntarily increase tongue force using a tongue exercise program that incorporates dynamic resistance to a load, and also that tongue exercise is associated with morphometric changes within the GG muscle.
Voluntary tongue force maxima were not significantly reduced prior to exercise in the old versus the young adult or middle-aged rats. As such, we did not find baseline levels of tongue weakness associated with old age in our rat subjects, as has been reported for elderly humans (Nicosia et al., 2000). However, the fact that voluntary tongue forces were not significantly different at baseline across age groups is consistent with prior research in the rat orolingual system investigating licking behavior (Stanford et al., 2003). Peak tongue force in the study by Stanford et al. (2003) was approximately 2.8 g for the young rats and 3.4 g for the older (24 mo. old) rats, a difference that was not statistically significant. The baseline forces in the current report were in a comparable range. Accordingly, the tongue forces used for licking or pressing the tongue against a disk to obtain water, with a progressively increasing load resistance, were not impaired with aging. An aging effect was also not seen in GG muscle fiber cross sectional area. Specifically, GG muscle fiber atrophy was not found with age in the rat model we employed, in contrast to the findings of prior studies in human intrinsic tongue muscles (Nakayama, 1991) and of the Wistar rat GG muscles (Oliven et al., 2001). Species, strain, muscle and methodology differences may account for these disparate results and further exploration is needed.
Following the tongue exercise program, maximum voluntary tongue force was significantly higher in all age groups. The young adult rats, however, manifested greater gains in force than the old rats. As such, young adult animals successfully adapted force levels in accordance with task requirements to a greater extent than old animals. These results suggest that tongue actions may be less amenable to training in old animals, with a smaller dynamic range for force generation. While a large dynamic range for strength may not impair performance of tasks that require less than the maximum, there has been discussion in the literature of the notion of a “physical reserve,” for use in maintaining physical function during or after illness or injury (Galvao and Taaffe, 2005). In this manner, increasing maximum voluntary force levels for craniofacial muscles may allow for greater strength and endurance by increasing resistance to fatigue.
While voluntary maximal tongue forces (MVTF) at baseline were not affected by aging, other work from our laboratory has shown that tetanic forces evoked by supramaximal stimulation of the hypoglossal nerves were reduced with aging for protrusive muscle actions (Nagai et al., in press). Specifically, in old animals, the average tetanic forces were in the area of 10 g, while young adult animals averaged 15–17 g of tetanic force with supramaximal stimulation of the medial branch of the hygoglossal nerve. Following exercise in the current study, the average MVTF for the young adult animals was approximately 7 g, thus not reaching tetanic force levels for the tongue protrusion observerd in our prior study (Nagai et al., in press). These findings suggest that while maximum capacity of the tongue for protrusive force may be reduced with aging, the tongue force levels required for the exercise task employed by this study were not affected by aging.
Animal age did not affect the number of tongue presses at baseline or following the tongue exercise program. A prior study (Stanford et al., 2003) found that the number of licks was increased in 24-month old animals when compared with 6-month old animals, a finding not replicated in this study. Differences in the rat strain and study methods may account for this difference in findings. However, the number of tongue presses post-exercise was significantly greater than baseline levels across age groups. The increase in the number of tongue presses, in combination with greater force levels post-exercise could be indicative of increased endurance as a function of exercise, or it could reflect that rats were simply better at performing the task after 8 weeks of experience with the operandum. That is, the increased force levels and number of tongue presses obtained post-exercise could reflect an improvement in task participation due to learning. Our experimental design did not allow us to distinguish between increased force levels and number of tongue presses due to increased tongue strength and/or endurance versus learning.
The tongue exercise paradigm used in the present study was intended to approximate research and treatment used in humans in which progressive resistance exercise of the tongue is employed (Robbins et al., 2005, 2007). Force outcomes postexercise in the rats were similar to the findings of tongue exercise protocols in humans in that increased tongue force was observed following 8 weeks of exercise (Robbins et al., 2005, 2007). However, there are several differences between the exercise model used in this study and the exercise task used in prior human studies of tongue exercise. For instance, clinical paradigms that employ progressive resistance exercise of the tongue using the Iowa Oral Performance Instrument (IOPI) or tongue depressors are isometric in nature. On the other hand, the rats in our study employed a more dynamic or isotonic tongue action, but with progressively increasing force requrements for a water reward.
The isotonic tongue action performed in the tongue exercise program may have had the characteristics of both an endurance task and a resistance task, rather than the solely progressive resistance exercise used in previous human clincial implementations (Robbins et al., 2005, 2007). With progressive resistance, a load is set at progressively increasing levels based upon maximum force capacity, and a relatively small number of repetitions are generally produced at these high force levels (Kraemer et al., 2002). The rats in this study produced a much larger number of tongue presses per session than the 3 sets of 8–10 presses typically used in strength training exercises for upper and lower body muscle groups. Based on previous recordings of tetanic force levels in the rat GG elicited by supramaximal stimulation (Nagai et al., in press), the load used in this study may be best characterized as a moderate load resistance. As noted, our procedure was to evaluate the EMP based on voluntary behavioral data and thus may not have approached a physiologic maximum. Therefore, the tongue exercise program used in the current study may not have faciliated muscle hypertrophy to the same extent due to this endurance component. The finding of moderate, but not significant, increases in GG muscle cross sectional area reported in this study may therefore be explained by the nature of the task performed by the rats. The use of a task that addresses endurance, and also strength, appears approprate for the treatment or prevention of age-related dysphagia. Because swallows of a food bolus rarely occur in isolation, eating a meal has been referred to as an endurance task (cf. Kays and Robbins, 2006).
The issue of tongue strength versus endurance was studied previously via lingual pressure measurements in human subjects who use the tongue normally versus “supranormal” tongue users; namely, trumpet players and debaters (Robin et al., 1992). Results indicated that maximum tongue pressures were not significantly different in supranormals versus normals. As such, there was not a difference on a measure of tongue strength. However, tongue endurance was signifcantly greater in the supranormals. Therefore, the greater skill level and frequent tongue exercise employed by the supranormals was associated with reduced fatiguability of the tongue. These results suggest that tongue exercise may be associated with increased endurance, and could be examined in clinical populations undergoing tongue exercise programs.
The findings of this work may have implications for the treatment of elderly persons with dysphagia using progressive resistance exercise of the tongue. Specifically, increases in tongue force that occur following 8 weeks of tongue exercise may be accompanied by alterations in tongue muscle fiber morphology. These changes may provide greater strength and endurance for goal-oriented actions associated with the oropharyngeal stages of the swallow and should be specifically examined in future research.
Acknowledgments
The authors are grateful for the assistance of Kyungah Lee, Scott Speer, Kiara Marlega, Emily Behea, Aaron Johnson, Glen Leverson, Alejandro Munoz del Rio, Karen Williams, Rachel Nelson, Sarah Cain, Hayley Gallaher, and Lisa Vinney in the completion of this work. Drs. Michelle Ciucci and JoAnne Robbins provided valuable comments on an earlier draft of this paper. This study was supported by grants from the National Institute of Deafness and Other Communication Disorders (R01DC005935 and R01DC008149).
References
- Balice-Gordon RJ. Age-related changes in neuromuscular innervation. Muscle & Nerve. 1997;5:S83–S87. doi: 10.1002/(sici)1097-4598(1997)5+<83::aid-mus20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Bodner L. Aging and oral motor function: evidence for altered performance among older persons. Journal of Dental Research. 1983;62:2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- Buchman AS, Boyle PA, Wilson RS, Bienias JL, Bennett DA. Physical activity and motor decline in older persons. Muscle & Nerve. 2007;35 doi: 10.1002/mus.20702. [DOI] [PubMed] [Google Scholar]
- Clark HM, Henson PA, Barber WD, Stierwalt JAG, Sherrill M. Relationships among subjective and objective measures of tongue strength and oral phase swallowing impairments. American Journal of Speech-Language Pathology. 2003;12:40–50. doi: 10.1044/1058-0360(2003/051). [DOI] [PubMed] [Google Scholar]
- Coles S. An Introduction to Statistical Modeling of Extreme Values. New York: Springer; 2001. [Google Scholar]
- Connor NP, Suzuki T, Lee K, Sewall GK, Heisey DM. Neuromuscular junction changes in aged rat thyroarytenoid muscle. Annals of Otology, Rhinology, and Laryngology. 2002;111:579–586. doi: 10.1177/000348940211100703. [DOI] [PubMed] [Google Scholar]
- Doherty TJ. Aging and Sarcopenia. Journal of Applied Physiology. 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Ekberg O, Feinberg MJ. Altered swallowing function in elderly patients without dysphagia: radiologic findings in 56 cases. AJR American Journal of Roentgenology. 1991;156:1181–1184. doi: 10.2214/ajr.156.6.2028863. [DOI] [PubMed] [Google Scholar]
- El Sharkawi A, Ramig L, Logemann JA, Pauloski BR, Rademaker AW, Smith CH, Pawlas A, Baum S, Werner C. Swallowing and voice effects of Lee Silverman Voice Treatment (LSVT[R]): a pilot study. Journal of Neurology, Neurosurgery, and Psychiatry. 2002;72:31–36. doi: 10.1136/jnnp.72.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiatarone MA, Marks EC, Ryan ND, Meredith CN, Lipsitz LA, Evans WJ. High intensity strength training in non-agenarians. Effects on skeletal muscle. JAMA. 1990;263:3029–3034. [PubMed] [Google Scholar]
- Fowler SC, Mortell C. Low doses of haloperidol interfere with rat tongue extensions during licking: A quantitative analysis. Behavioral Neuroscience. 1992;106:386–395. doi: 10.1037//0735-7044.106.2.386. [DOI] [PubMed] [Google Scholar]
- Frontera WR, Meredith CN, O’Reilly KP, Knuttgen HG, Evans WJ. Strength conditioning in older men: Skeletal muscle hypertrophy and improved function. Journal of Applied Physiology. 1988;64:1038–1044. doi: 10.1152/jappl.1988.64.3.1038. [DOI] [PubMed] [Google Scholar]
- Galvao DA, Taaffe DR. Resistance exercise dosage in older adults: single- versus multiset effects on physical performance and body composition. Journal of the American Geriatrics Society. 2005;53:2090–2097. doi: 10.1111/j.1532-5415.2005.00494.x. [DOI] [PubMed] [Google Scholar]
- Grimby G, Aniansson A, Hedberg M, Henning G-B, Grangard U, Kvist H. Training can improve muscle strength and endurance in 78–84 year old men. Journal of Applied Physiology. 1992;73:2517–2523. doi: 10.1152/jappl.1992.73.6.2517. [DOI] [PubMed] [Google Scholar]
- Kays S, Robbins J. Effects of sensorimotor exercise on swallowing outcomes relative to age and age-related disease. Seminars in Speech and Language. 2006;27:245–259. doi: 10.1055/s-2006-955115. [DOI] [PubMed] [Google Scholar]
- Kraemer WJ, Adams K, Cafarelli E, Dudley GA, Dooly C, Feigenbaum MS, Fleck SJ, Franklin B, Fry AC, Hoffman JR, Newton RU, Potteiger J, Stone MH, Ratamess NA, Triplett-McBride T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Medicine and Science in Sports and Exercise. 2002;34:364–380. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- Lazarus C, Logemann JA, Huang CF, Rademaker AW. Effects of two types of tongue strengthening exercises in young normals. Folia Phoniatrica et Logopaedica. 2003;55:199–205. doi: 10.1159/000071019. [DOI] [PubMed] [Google Scholar]
- Lazarus CL, Logemann JA, Pauloski BR, Colangelo LA, Kahrilas PJ, Mittal BB, Pierce M. Swallowing disorders in head and neck cancer patients treated with radiotherapy and adjuvant chemotherapy. Laryngoscope. 1996;106:1157–1166. doi: 10.1097/00005537-199609000-00021. [DOI] [PubMed] [Google Scholar]
- Lazarus CL, Logemann JA, Pauloski BR, Rademaker AW, Larson CR, Mittal BB, Pierce M. Swallowing and tongue function following treatment for oral and oropharyngeal cancer. Journal of Speech, Language, and Hearing Research. 2000;43:1011–1023. doi: 10.1044/jslhr.4304.1011. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH. Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. Journal of Speech and Hearing Research. 2000;43:1264–1274. doi: 10.1044/jslhr.4305.1264. [DOI] [PubMed] [Google Scholar]
- McKee GJ, Johnston BT, McBride GB, Primrose WJ. Does age or sex affect pharyngeal swallowing? Clin Otolaryngol. 1998;23:100–106. doi: 10.1046/j.1365-2273.1998.00100.x. [DOI] [PubMed] [Google Scholar]
- Mortimore IL, Fiddes P, Stephens S, Douglas NJ. Tongue protrusion force and fatiguability in male and female subjects. European Respiratory Journal. 1999;14:191–195. doi: 10.1034/j.1399-3003.1999.14a32.x. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Birkestrand B, Fowler SC. The neuroimmunophilin GPI-1046 paritally protects against 3-acetylpyridine toxicity in the rat. Neuroscience Letters. 2002;321:53–56. doi: 10.1016/s0304-3940(01)02571-x. [DOI] [PubMed] [Google Scholar]
- Moss SJ, Wang G, Chen R, Pal R, Fowler SC. 3-Acetylpyridine reduces tongue protrusion force but does not abolish lick rhythm in the rat. Brain Research. 2001;920:1–9. doi: 10.1016/s0006-8993(01)02790-1. [DOI] [PubMed] [Google Scholar]
- Nagai H, Russell JA, Jackson MA, Connor NP. Effect of aging on tongue protrusion forces in rats. Dysphagia. doi: 10.1007/s00455-007-9103-6. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M. Histological study on aging changes in the human tongue. J Otolaryngol Japan. 1991;94:541–555. doi: 10.3950/jibiinkoka.94.541. [DOI] [PubMed] [Google Scholar]
- Nicosia MA, Hind JA, Roecker EB, Carnes M, Doyle J, Dengel GA, Robbins J. Age effects on the temporal evolution of isometric and swallowing pressure. Journals of Gerontology Series A, Biological Sciences and Medical Sciences. 2000;55:M634–M640. doi: 10.1093/gerona/55.11.m634. [DOI] [PubMed] [Google Scholar]
- Oliven A, Carmi N, Coleman R, Majed O, Silbermann M. Age-related changes in upper airway muscles morphological and oxidative properties. Experimental Gerontology. 2001;36:1673–1686. doi: 10.1016/s0531-5565(01)00127-9. [DOI] [PubMed] [Google Scholar]
- Robbins J, Butler SG, Daniels SK, Gross RD, Langmore S, Lazarus CL, Martin-Harris B, McCabe D, Musson N, Rosenbek JC. Swallowing and dysphagia rehabiliation: Translating principles of neural plasticity into clinically oriented evidence. Journal of Speech, Language, and Hearing Research. 2008;51:S276–S300. doi: 10.1044/1092-4388(2008/021). [DOI] [PubMed] [Google Scholar]
- Robbins J, Kays SA, Gangnon RE, Hind JA, Hewitt AL, Gentry LR, Taylor AJ. The effects of lingual exercise in stroke patients with dysphagia. Archives of Physical Medicine and Rehabilitation. 2007;88:150–158. doi: 10.1016/j.apmr.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Robbins J, Levine R, Wood J, Roecker ED, Luschei E. Age effects on lingual pressure generation as a risk factor for dysphagia. Journal of Gerontology. 1995;50:M257–M262. doi: 10.1093/gerona/50a.5.m257. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Gangnon RE, Theis SM, Kays SA, Hewitt AL, Hind J. The Effects of Lingual Exercise on Swallowing in Older Adults. Journal of the American Geriatrics Society. 2005;53:1493–1489. doi: 10.1111/j.1532-5415.2005.53467.x. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Hamilton JW, Lot GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology. 1992;103:823–829. doi: 10.1016/0016-5085(92)90013-o. [DOI] [PubMed] [Google Scholar]
- Robin DA, Goel A, Somodi LB, Luschei ES. Tongue strength and endurance: Relation to highly skilled movements. Journal of Speech and Hearing Research. 1992;35:1239–1245. doi: 10.1044/jshr.3506.1239. [DOI] [PubMed] [Google Scholar]
- Rosenberg RH. Sarcopenia: Origins and clinical relevance. Journal of Nutrition. 1997;127:990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- Rosenheimer JL. Ultraterminal sprouting in innervated and partially denervated adult and aged rat muscle. Neuroscience. 1990;38:763–770. doi: 10.1016/0306-4522(90)90069-g. [DOI] [PubMed] [Google Scholar]
- Shaker R, Kern M, Bardan E, Taylor A, Stewart ET, Hoffmann RG, Arndorfer RC, Hofmann C, Bonnevier J. Augmentation of deglutitive upper esophageal sphincter opening in the elderly by exercise. American Journal of Physiology. 1997;35:G1518–G1522. doi: 10.1152/ajpgi.1997.272.6.G1518. [DOI] [PubMed] [Google Scholar]
- Smittkamp SE, Brown JW, Stanford JA. Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience. 2008;151:613–621. doi: 10.1016/j.neuroscience.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged F344 rats exhibit altered orolingual motor function: relationships with nigrostriatal neurochemical measures. Neurobiology of Aging. 2003;24:259–266. doi: 10.1016/s0197-4580(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Stathopoulos E, Duchan JF. History and principles of exercise-based therapy: How they inform our current treatment. Seminars in Speech and Language. 2006;27:227–235. doi: 10.1055/s-2006-955113. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Duret C, Wheeler S, Marcus R. Once-weekly resistance exercise improves muscle strength and neuromuscular performance in older adults. Journal of the American Geriatrics Society. 1999;47:1208–1214. doi: 10.1111/j.1532-5415.1999.tb05201.x. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Pruitt L, Pyka G, Guido D, Marcus R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clinical Physiology. 1996;16:381–392. doi: 10.1111/j.1475-097x.1996.tb00727.x. [DOI] [PubMed] [Google Scholar]
- Toth LA, Gardiner TW. Food and water restriction protocols: Physiological and behavioral considerations. Contemporary Topics in Laboratory Animal Science. 2000;39:9–17. [PubMed] [Google Scholar]
- Tracy BL, Ivey FM, Hurlbut D, Martel GF, Lemmer JT, Siegel EL, Metter EJ, Fozard JL, Fleg JL, Hurley BF. Muscle quality. II. Effects Of strength training in 65- to 75-yr-old men and women. Journal of Applied Physiology. 1999;86:195–201. doi: 10.1152/jappl.1999.86.1.195. [DOI] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the biomarkers of aging program. J Gerontol [A] Biol Sci Med Sci [A] Biol S. 1999;54A:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Vandervoort AA. Aging of the human neuromuscular system. Muscle & Nerve. 2002;25:17–25. doi: 10.1002/mus.1215. [DOI] [PubMed] [Google Scholar]
- Vincent KR, Braith RW, Feldman RA, Magyari PM, Cutler RB, Persin SA, Lennon SL, Gabr AH, Lowenthal DT. Resistance exercise and physical performance in adults aged 60 to 83. Journal of the American Geriatrics Society. 2002;50:1100–1107. doi: 10.1046/j.1532-5415.2002.50267.x. [DOI] [PubMed] [Google Scholar]