Fig. 1.
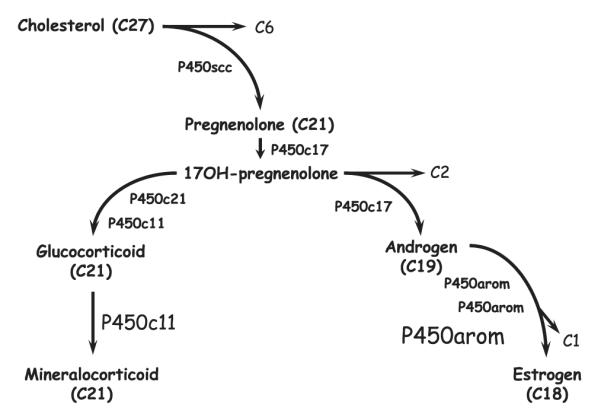
Schematic representation of the steroidogenic cascade involving the steroid hydroxylating cytochromes P450 (P450) that catalyze cleavage and/or hydroxylation of sequential substrates from cholesterol through pregnenolone, androstane and estrane (synthesis). The enzymes catalyzing each step are shown; cholesterol side chain cleavage (P450scc) cleaves the 6 carbon side chain (C6) from the 27 carbon skeleton (C27) releasing pregnenolone (C21). Subsequent metabolism by 17α-hydroxylase/17,20-lyase (P450c17) produces 17-hydroxypregnanes (C21) that are intermediates in the production of either corticoids or sex steroids. Metabolism of 17-hydroxypregnanes by 21-hydroxylase (P450c21) and 11β-hydroxylase (P450c11) directs the pathway toward corticoid production. Alternatively, cleavage of the 17-hydroxypregnanes in a second oxidation catalyzed by P450c17 directs the pathway to androgen (C19) synthesis and then, after three oxidations culminating in cleavage of a methyl, to estrogens (C18). Note that P450c11 and P450arom represent the terminal enzymes in the steroid synthetic cascade.
