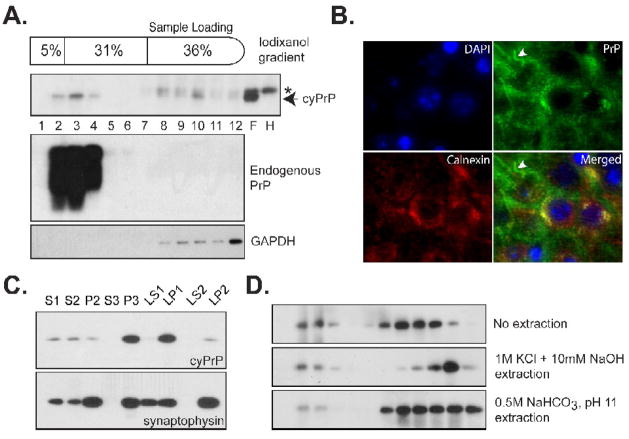
Fig. 6. The interaction between cyPrP and lipid membranes.
(A) Post-nuclear supernatant prepared from the forebrain of an induced heterozygous transgenic mouse (pBI-cyPrP-Gal+/−; tTA+/−) was separated by the iodixanol gradient. The presence of cyPrP in each fraction was detected by the 3F4 antibody. The same blot was re-probed by POM1 anti-PrP antibody to identify membrane associated endogenous PrP, and an anti-GAPDH antibody to identify cytosolic GAPDH protein. Number represents fractions from top to the bottom. F, forebrain; H, hindbrain; Arrow indicates the position of cyPrP; Asterisk indicates a non-specific reactive band that was present in both forebrain and hindbrain lysates. (B) CA1 region of a transgenic mouse brain was stained with antibodies against cyPrP (green) and ER membrane protein calnexin (red). Nuclei were stained with DAPI (blue). Yellow color in the merged image indicates the co-localization between calnexin and cyPrP in the cell body. White arrowhead points to cyPrP in the neuronal process that is not co-localized with calnexin. (C) The presence of cyPrP in different neuronal subcellular membranes. CyPrP and synaptophysin were detected by immunoblot analysis, and the total protein of each fraction is 10μg. (D) Post-nuclear supernatant prepared from an induced homozygous transgenic mouse were subjected to no extraction, extraction with 1M KCl plus 10mM NaOH or with 0.5M NaHCO3, pH 11, and then separated by the iodixanol gradient. CyPrP was detected by immunoblot analysis with 3F4 antibody.