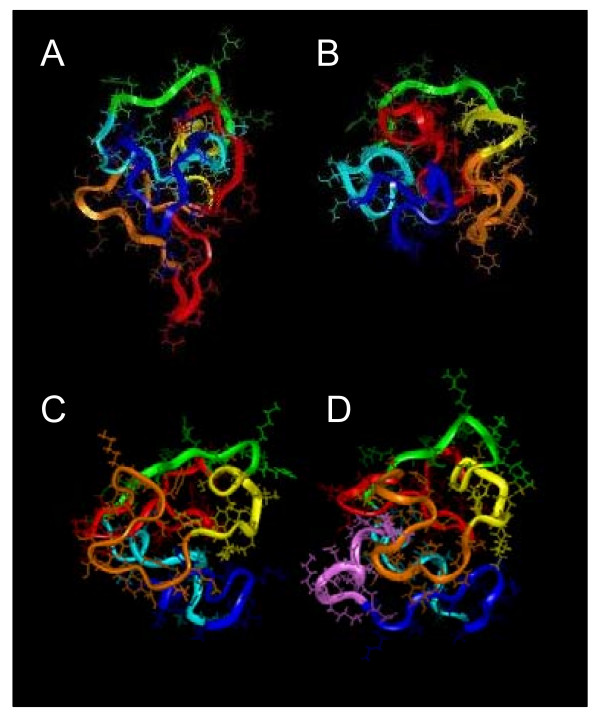
Figure 3.
NMR studies of Tat proteins. Tat Z2 (A), Tat Bru (B), Tat Mal (C), and Tat Eli (D) 3D structures obtained from NMR constraints [36-38,44]. Region I is depicted in red, region II (cysteine-rich region) in orange, region III in yellow, region IV (basic region) in green, region V in light blue, region VI (residues 73–86/87) in blue and for Tat Eli the extra C-terminal residues are in pink. The Tat Z2 variant used had chemically modified cysteines which affected biological activity and 3D structure. The three Tat variants with biological activity (B, C and D) displayed a similar folding characterized by a core region composed of part of region I with the highly conserved Trp11 while the functional region II, IV and V are well exposed to the solvent. The extra residues in the C-terminus of Tat Eli are exposed to the solvent and protrude from a groove between the basic region and the cysteine-rich region.