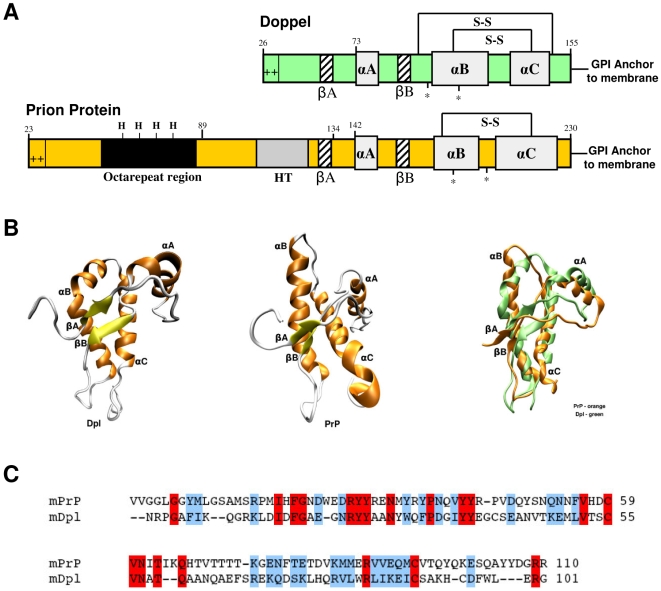
Figure 1. Mature PrP and Dpl protein share common structural architectures.
(A) PrPC and Dpl have common secondary structure elements, composed by three alpha helices (αA, αB and αC) and two beta strands (βA and βB). Both PrPC and Dpl have N-glycosylation sites (*), disulfide bridges (S-S) and a GPI-moiety, which links the proteins to the extracellular side of the cellular membrane. PrPC and Dpl also share a positively charged N-terminus. PrPC contains five octapeptide repeats capable of binding copper through histidine residues (modified from [52]). (B) The topology of Dpl (PDB code: 1I17, left structure) is very similar to that of PrPC (PDB code: 1AG2, central structure). A significant difference is that αB helix of Dpl (in green, right image) is bent and that the two beta strands are oriented differently than those in the PrPC (in orange, right image). (C) Sequence alignment between mouse PrPC (mPrP, residues Val120–Arg229; SwissProt entry: P04925) and mouse Dpl (mDpl, residues Asn55–Gly155; SwissProt entry: Q9QUG3) proteins. In this tract the two proteins share 18% of sequence identity and 44% of sequence similarity. Fully conserved residues are highlighted in red, while semi-conserved are shown in blue.