Abstract
AIM: To study the effect of aprotinin used in orthotopic liver transplantation (OLT) on the intraoperative requirement for blood products and on the incidence of laparotomy for bleeding, thrombotic events and mortality.
METHODS: A systematic review of the literature in the electronic database Medline and the Clinic Trials Registry Database was performed. Literature that did not fit our study were excluded. Patients in the reviewed studies were divided into two groups; one group used aprotinin (aprotinin group) while the other did not (control group). The data in the literature that fit our requirements were recorded. Weighted mean differences (WMD) in the requirements for blood products between the aprotinin group and the control group were tested using a fixed effect model. A Z test was performed to examine their reliability; the Fleiss method of fixed effect model was used to analyze data on postoperative events, and odds ratios (ORs) were tested and merged.
RESULTS: Seven citations were examined in our study. Among them, a requirement for blood products was reported in 4 studies including 321 patients, while postoperative events were reported in 5 studies including 477 patients. The requirement for red blood cells and fresh frozen plasma in the aprotinin group was statistically lower than that in the control group (WMD = -1.80 units, 95% CI, -3.38 to -0.22; WMD = -3.99 units, 95% CI, -6.47 to -1.50, respectively). However, no significant difference was indicated in the incidence of laparotomy for bleeding, thrombotic events and mortality between the two groups. Analysis on blood loss, anaphylactic reactions and renal function was not performed in this study due to a lack of sufficient information.
CONCLUSION: Aprotinin can reduce the intraoperative requirement for blood products in OLT, and has no significant effect on the incidence of laparotomy for bleeding, thrombotic events and mortality.
Keywords: Aprotinin, Liver transplantation, Blood transfusion, Meta-analysis
INTRODUCTION
Orthotopic liver transplantation (OLT) has become the first choice approach for the treatment of patients with end-stage liver diseases[1]. However, despite great improvements in graft preservation, surgical skills, anesthetic techniques and perioperative management[2,3], OLT is still associated with severe bleeding and considerable transfusion requirements, which in turn greatly contribute to the peri-operative morbidity and mortality[4]. Severe bleeding in OLT occurs for several reasons, among which hemostatic abnormalities remain a major cause[5,6].
Aprotinin, a serine protease inhibitor, is more and more commonly being used in surgeries, such as cardiac surgeries and liver transplantations, to reduce bleeding and the need for transfusions. A meta-analysis of 12 trials (n = 626) of children undergoing cardiac surgery demonstrated aprotinin reduced the proportion of children receiving blood transfusions during cardiac surgery with cardiopulmonary bypass, but had no significant effect on the volume of blood transfused or on the amount of chest tube drainage[7]. Similarly, a meta analysis of 13 trials (n = 506) of patients undergoing major orthopedic surgery demonstrated the pooled blood loss and the amounts of red blood cell (RBC) units (U) transfused intraoperatively and peri-operatively were significantly lower among aprotinin-treated patients than control patients. Moreover, aprotinin was not associated with an increased incidence of deep vein thrombosis[8]. However, there are still some conflicting results on whether aprotinin can reduce blood loss or the requirement for transfusion in OLT[9,10], and whether it can be beneficial to postoperative outcomes[11,12]. The objective of this systemic review was to study the effect of aprotinin used in OLT on the intraoperative requirement of blood products, and on the incidence of laparotomy for bleeding, thrombotic events and mortality.
MATERIALS AND METHODS
Data source
We searched the electronic database of Medline and the Clinic Trials Registry Database using aprotinin and liver transplantation as keywords. References cited by other retrospective articles and related articles or summaries from foreign journals were searched manually as well. After initial screening, we examined the titles and abstracts of potentially eligible trials, and selected those which met the following predefined inclusion criteria: published clinical controlled trials on the use of aprotinin in liver transplantation, English language, adult study population, with data on (1) the transfusion requirement for blood products, (2) perioperative mortality and morbidity, (3) incidence of postoperative thrombotic events and (4) incidence of laparotomy for bleeding. Citations that did not fit our study or contained insufficient information were excluded.
Statistical analysis
We recorded the data that fit our requirements, examined their heterogeneity, and calculated the weighted mean difference (WMD) or odds ratio (OR) between the two groups. All calculations were performed using the software Review Manager 4.2 (The Nordic Cochrane Centre, The Cochrane Collaboration 2003, Copenhagen, Denmark).
We used the difference of means (yi) as the effect scale of the data on requirements of blood products and examined their heterogeneity (Q < χ2 (0.05, k-1), P > 0.05), if P > 0.05, fixed effect model was used to calculate WMD and 95% confidence interval (95% CI); otherwise, a random effect model was used. If the 95% CI included 0, then there was no significant difference between the two groups. However, if the 95% CI was greater than 0, then the control group was supported; otherwise, the aprotinin group was supported.
We calculated the ORs of the incidence of mortality, laparotomy for bleeding and thrombotic events, and tested their heterogeneity (Q < χ2 (0.05, k-1), P > 0.05). If P > 0.05, the homogeneity was considered good, and a fixed effect model was used to calculate the total OR and 95% CI; otherwise a random effect model was chosen. If 1 was included in the 95% CI, then there was no statistically significant difference between the groups. If the 95% CI was more than 1, the control group was supported; otherwise, the aprotinin group was supported.
RESULTS
Recording of data
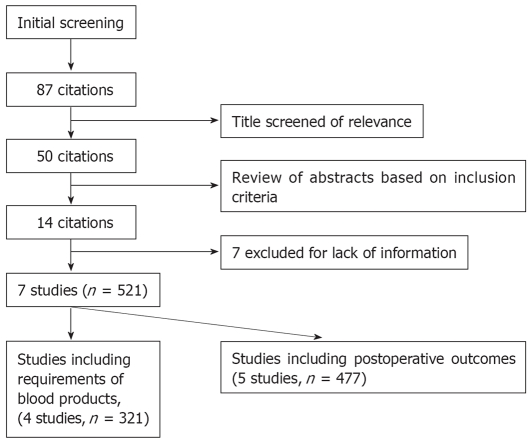
We identified 87 citations in a primary literature search. Titles were screened for relevance, eliminating 37 citations, and then abstracts and contents were read carefully, leading to the exclusion of a further 36 citations; 7 more were excluded because of a lack of information. Finally 7 citations[9,10,13–17], including 521 patients, were included in our study (Figure 1). Of these 7 studies, one used tranexamic acid in the control group[16]; two studies contained two aprotinin groups, a high dose group and a routine dose group[10,15]; one study contained two control groups[17]; and two studies used the same sample[10,15], the size of which was calculated only once.
Figure 1.
Results of article search and selection.
Effect of aprotinin on RBC requirement
Four citations, including 321 procedures, contained results on the requirement for blood products including RBCs and fresh frozen plasma (FFP)[10,14,16,17].
One of these studies contained two control groups[17] (C1 and C2); Neither aprotinin nor any other antifibrinolytic agent was used in either group, so we just took C2 as the control group. Heterogeneity was tested: Q = 8.87, γ = 3, χ2 (0.05, 3) = 7.81, P < 0.05. As the homogeneity was low, a random effect model was used: WMD = -1.23 units, 95% CI, -3.17 to 0.71; no statistical significance was indicated. Considering one study used tranexamic acid in control group[17], it perhaps influenced the veracity, so we excluded that study and tested again, Q = 3.85, γ = 2, χ2(0.05, 2) = 5.99, P > 0.05, calculated with fixed effect model. It was indicated the intraoperative requirement for RBCs was significantly lower in the aprotinin group than the control group (WMD = -1.80 units, 95% CI -3.38 to -0.22; Table 1A and 1B).
Table 1A.
Volumes of RBCs transfused intraoperatively in the 4 studies
| Study |
Aprotinin group |
Control group |
yi (95% CI) | ||||
| Mean | SD | n | Mean | SD | n | ||
| (units) | (units) | (units) | (units) | ||||
| 1 Garcia HL | 13.0 | 8.0 | 39 | 14.4 | 9.7 | 41 | -1.4 (-5.29, 2.49) |
| 2 Marcel RJ | 2.1 | 2.0 | 21 | 3.0 | 4.4 | 23 | -0.9 (-2.89, 1.09) |
| 3 Dalmau A1 | 2.44 | 3.03 | 63 | 2.14 | 2.32 | 64 | 0.3 (-0.64, 1.24) |
| 4 Llamas P | 8.1 | 5.2 | 20 | 13 | 7.4 | 30 | -4.9 (-8.39, -1.41) |
Control group used tranexamic acid.
Table 1B.
Weighted mean differences (WMDs) in the volumes of RBCs transfused intraoperatively
|
Heterogeneity |
WMD |
Z test |
95% CI | |||
| Q | P | (units) | Z | P | ||
| A | 8.87 | 0.03 | -1.23 | 1.24 | 0.21 | -3.17 to 0.71 |
| B | 3.85 | 0.15 | -1.80 | 2.23 | 0.03 | -3.38 to -0.22 |
A: Total results; B: Results with study No. 3 excluded.
Effect of aprotinin on FFP requirement
The heterogeneity of the 4 citations was low (Q = 13.77, γ = 3, χ2(0.05, 3) = 7.81, P < 0.05), so a random effect model was used. No significant difference was indicated (WMD = -3.13 units, 95% CI -6.79 to 0.53). If the study using individuals treated with tranexamic acid as a control group was excluded, the heterogeneity was better (Q = 5.25, χ2 (0.05, 2) = 5.99, P > 0.05), and a fixed effect model was chosen. It was indicated the intraoperative requirement for FFP was significantly lower in the aprotinin group than in the control group (WMD = -3.99 units, 95% CI -6.47 to -1.50; Table 2A and 2B).
Table 2A.
Volumes of FFP transfused intraoperatively in the 4 studies
| Study |
Aprotinin group |
Control group |
yi (95% CI) | ||||
| Mean | SD | n | Mean | SD | n | ||
| (units) | (units) | (units) | (units) | ||||
| 1 Garcia HL | 26.0 | 16.0 | 39 | 28.0 | 15.0 | 41 | -2.00 (-8.80, 4.80) |
| 2 Marcel RJ | 3.6 | 3.5 | 21 | 6.6 | 6.1 | 23 | -3.00 (-5.91, -0.09) |
| 3 Dalmau A1 | 1.09 | 2.20 | 63 | 1.20 | 2.21 | 64 | -0.11 (-0.88, 0.66) |
| 4 Llamas P | 16.7 | 10.4 | 20 | 28 | 14 | 30 | -11.3 (-18.07, -4.53) |
Control group used tranexamic acid.
Table 2B.
Weighted mean differences (WMDs) in the volumes of FFP transfused intraoperatively
|
Heterogeneity |
WMD |
Z test |
95% CI | |||
| Q | P | (units) | Z | P | ||
| A | 13.77 | 0.003 | -3.13 | 1.68 | 0.09 | -6.79 to 0.53 |
| B | 5.25 | 0.07 | -3.99 | 3.14 | 0.002 | -6.47 to -1.50 |
A: Total results; B: Results with study No. 3 excluded.
Effect of aprotinin on postoperative outcomes
As can be seen from Table 3A and 3B, no significant difference was indicated in the incidence of laparotomy for bleeding, thromboembolic events and mortality between the two groups.
Table 3A.
Postoperative outcomes of the 5 studies
| Study |
Aprotinin group |
Control group |
ORi1, 95% CI | ORi2, 95% CI | ORi3, 95% CI | ||||||
| n | n1 | n2 | n3 | n | n1 | n2 | n3 | ||||
| 1 James Y | 33 | 0 | 1 | 1 | 30 | 1 | 4 | 2 | 0.29 (0.01, 7.48) | 0.20 (0.02, 1.93) | 0.44 (0.04, 5.09) |
| 2 Garcia HL | 39 | 1 | 2 | 41 | 1 | 3 | 1.05 (0.06, 17.43) | 0.68 (0.11, 4.34) | |||
| 3 Porte RJ | 89 | 5 | 7 | 2 | 48 | 5 | 2 | 3 | 0.51 (0.14, 1.87) | 1.96 (0.39, 9.85) | 0.34 (0.06, 2.14) |
| 4 Dalmau A1 | 63 | 1 | 2 | 2 | 64 | 4 | 2 | 4 | 0.24 (0.03, 2.23) | 1.02 (0.14, 7.45) | 0.49 (0.09, 2.79) |
| 5 Llamas P | 20 | 4 | 0 | 50 | 6 | 7 | 1.83 (0.46, 7.35) | 0.14 (0.01, 2.60) | |||
n1: Number of deaths; n2: Number of laparotomy for bleeding; n3: Number of thromboembolic events;
Control group used tranexamic acid.
Table 3B.
Odds ratios of the postoperative outcomes
|
Heterogeneity of ORi |
OR |
χ2 test |
95% CI | ||||
| Q | P | χ2 | P | ||||
| Death | A | 3.32 | 0.51 | 0.69 | 0.48 | > 0.05 | 0.32-1.52 |
| B | 2.21 | 0.53 | 0.85 | 0.06 | > 0.05 | 0.36-2.00 | |
| Laparotomy for bleeding | A | 4.09 | 0.39 | 0.65 | 0.44 | > 0.05 | 0.29-1.43 |
| B | 3.94 | 0.27 | 0.59 | 0.57 | > 0.05 | 0.25-1.41 | |
| Thrombotic events | A | 0.08 | 0.96 | 0.42 | 2.33 | > 0.05 | 0.14-1.30 |
| B | 0.02 | 0.88 | 0.38 | 1.75 | > 0.05 | 0.09-1.64 | |
A: Total results; B: Results with study No. 4 excluded.
DISCUSSION
Unlike traditional reviews, a meta analysis is a set of statistical procedures designed to accumulate experimental and correlational results across independent studies which address related sets of research questions. The aim of the meta-analysis is to determine a predefined inclusion criteria based on the systematic retrieval of literature on a given topic, and estimate the initial literatures carefully to ensure minimal bias in terms of the objectivity, validity and dependability of the results. The efficiency of the results depends on the choice of statistical method, as well as the rigidity of each study. In this meta analysis, we performed a wide search of the literature, identified as many studies as we could, and tested their heterogeneity (Q < χ2 (0.05, k-1), P > 0.05). If homogeneity was good (P > 0.05), we calculated data with a fixed effect model; otherwise we used a random effect model. Thus, we consider the statistical methods we used were correct and rigorous.
Several factors contribute to excessive bleeding during OLT, including pre-existing coagulopathy in patients[18–20], the procedure of liver transplantation itself, and the experience of the surgeon. However, hemostatic abnormalities due to hyperfibrinolysis remain a major cause. Hyperfibrinolysis always occurs late in the anhepatic phase and immediately after the reperfusion of the graft[21]. This enhanced fibrinolytic activation is due to an excess of tissue-type plasminogen activator (t-PA) on account of the lack of hepatic clearance and its increased release from the ischemically damaged endothelium, associated with the consumption of α2-antiplasmin (α2-AP) and plasminogen activator inhibitor type 1 (PAI 1)[22–24]. Using a suitable method to protect blood and control the coagulopathy of patients can not only reduce the need for transfusions, reduce the transmission of diseases and immunological reactions due to the transfusion of banked blood, but also have great benefit to peri-operative hemodynamic stability. Several methods can be used to protect blood during liver transplantation, including transfusion of autoblood, appropriate body temperature and perioperative use of blood protective drugs. Aprotinin, a basic polypeptide and non-specific proteinase inhibitor, can inhibit several proteases with serine active groups. Recently, aprotinin became no longer restricted to the treatment of patients with acute pancreatitis; it is being used more and more in cardiac surgeries, orthopedic surgeries and liver transplantations[25,26], and is considered the ideal blood protecting drug.
Aprotinin inhibits kallikrein, reduces the release of callidin and results in a decrease in the level of t-PA. In a larger, randomized, double-blind, placebo-controlled study, Molenaar et al[27] compared coagulation [fibrinogen level, activated partial thromboplastin time (aPTT), prothrombin time and platelet count] and fibrinolytic variables (tPA antigen and activity, plasminogen activator inhibitor activity and D-dimer), as well as thromboelastography results [reaction time (R), clot formation time, and maximum amplitude] between an aprotinin group and a placebo group. They found fibrinolytic activity (plasma D-dimer and tPA antigen levels) was significantly lower in aprotinin-treated patients compared with the placebo group, but coagulation times (aPTT and R) were significantly more prolonged. It was indicated aprotinin has an anticoagulant rather than a procoagulant effect. Its blood-sparing (prohemostatic) effect appears to be the overall result of a strong antifibrinolytic and a weaker anticoagulant effect. In our study, it was found that, when normal saline or a placebo was used in the control group, the requirement for red blood cells was significantly lower in the aprotinin group than in the control group (WMD = -1.80 units, 95% CI, -3.38 to -0.22 units; moreover, the requirement for fresh frozen plasma was also significantly lower in the aprotinin group than in the control group (WMD = -3.99 units, 95% CI, -6.47 to -1.50). However, no significant difference was indicated in the incidence of laparotomy for bleeding, thromboembolic events, and mortality between the two groups. It was demonstrated aprotinin can reduce the intraoperative requirement for blood products in OLT. The effect of aprotinin on blood loss during OLT was not reviewed in this study because of a lack of information; more clinic trials are needed for advanced investigation.
As an extraneous protein, aprotinin causes allergies, which can induce typical allergic reactions in patients[28,29], especially those who use it again. Aprotinin is mainly metabolized by the kidney, and it has nephrotoxicity if used at high doses. The serum creatinine levels in patients using aprotinin during an operation increased at 3-5 d postoperatively, indicating an influence on renal function[14]. However, it was also reported the number of patients whose serum creatinine levels increased by more than 5 mg/L was lower in the aprotinin group than in the control group. No significant difference was found in peri-operative creatinine clearance rates[30].
There are three causes of thromboembolic events: Injury of blood vessels, changes in the blood stream, and coagulation state. It was reported aprotinin can lead to intravascular thrombosis and thromboembolism during liver transplantation[31]. In our study, the OR of thrombosis was 0.42, the 95% CI was 0.14 to 1.30, and there was no significant deviation; While the study using TA as control group was excluded, the OR was 0.38, the 95% CI was 0.09 to 1.64, and there was also no statistically significant deviation. It appears aprotinin has no significant influence upon the incidence of thrombosis in patients undergoing liver transplantation, possibly due to its strong antifibrinolytic and a weaker anticoagulant effect.
As a statistical method for investigation, meta analysis has been used widely, but this method can not eliminate confounding factors and biases in each study, so the result could, unavoidably, include a certain bias. In our study, the dosages of aprotinin were different, and the drugs used in the control groups were not the same, all of which could contribute to bias. In addition, there still exists the “publish bias”; that is, articles that are published often have a tendency to have positive results, which could be decreased by collecting data that is as all-encompassing as possible.
Thus, aprotinin can reduce the intraoperative require-ment for blood products in OLT and has no significant effect on the incidence of laparotomy for bleeding, thromboembolic events and mortality. Of course, further clinical randomized controlled trials (RCTs) are needed to confirm this.
COMMENTS
Background
Orthotopic liver transplantation (OLT) is associated with severe bleeding and considerable transfusion requirements, while severe bleeding in OLT occurs for several reasons, among which hemostatic abnormalities remain a major cause.
Research frontiers
We performed a meta analysis to study the effect of aprotinin used in OLT on the intraoperative requirement for blood products and the postoperative outcomes.
Innovations and breakthroughs
This study clearly shows aprotinin can reduce the intraoperative requirement for blood products and has no significant effect on the incidence of laparotomy for bleeding, thromboembolic events and mortality.
Applications
Although additional clinical randomized controlled trials (RCTs) are required to clarify the role of aprotinin in OLT, this study strongly confirms the blood transfusion reducing effect of aprotinin, which has no significant effect on the incidence of laparotomy for bleeding, thromboembolic events and mortality.
Peer review
The authors investigated the effect of aprotinin used in OLT on the intraoperative requirement of blood products and on the incidence of laparotomy for bleeding, thrombotic events and mortality, using a systematic review of the literature. They concluded aprotinin can reduce the intraoperative requirement of blood product in OLT, and has no significant effect on the incidence of laparotomy for bleeding, thrombotic events and mortality.
Supported by Grant 02KJD320015 from the Education Committee of Jiangsu Province, China
Peer reviewer: Mitsuo Shimada, Professor, Department of Digestive and Pediatric Surgery, Tokushima University, Kuramoto 3-18-15, Tokushima 770-8503, Japan
S- Editor Yang RH L- Editor McGowan D E- Editor Ma WH
References
- 1.Starzl TE, Demetris AJ, Van Thiel D. Liver transplantation (1) N Engl J Med. 1989;321:1014–1022. doi: 10.1056/NEJM198910123211505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porte RJ, Hendriks HG, Slooff MJ. Blood conservation in liver transplantation: The role of aprotinin. J Cardiothorac Vasc Anesth. 2004;18:31S–37S. doi: 10.1053/j.jvca.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Ramos E, Dalmau A, Sabate A, Lama C, Llado L, Figueras J, Jaurrieta E. Intraoperative red blood cell transfusion in liver transplantation: influence on patient outcome, prediction of requirements, and measures to reduce them. Liver Transpl. 2003;9:1320–1327. doi: 10.1016/jlts.2003.50204. [DOI] [PubMed] [Google Scholar]
- 4.Bontempo FA, Lewis JH, Van Thiel DH, Spero JA, Ragni MV, Butler P, Israel L, Starzl TE. The relation of preoperative coagulation findings to diagnosis, blood usage, and survival in adult liver transplantation. Transplantation. 1985;39:532–536. doi: 10.1097/00007890-198505000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lewis JH, Bontempo FA, Awad SA, Kang YG, Kiss JE, Ragni MV, Spero JA, Starzl TE. Liver transplantation: intraoperative changes in coagulation factors in 100 first transplants. Hepatology. 1989;9:710–714. doi: 10.1002/hep.1840090509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation. 1989;47:978–984. doi: 10.1097/00007890-198906000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnold DM, Fergusson DA, Chan AK, Cook RJ, Fraser GA, Lim W, Blajchman MA, Cook DJ. Avoiding transfusions in children undergoing cardiac surgery: a meta-analysis of randomized trials of aprotinin. Anesth Analg. 2006;102:731–737. doi: 10.1213/01.ane.0000194954.64293.61. [DOI] [PubMed] [Google Scholar]
- 8.Shiga T, Wajima Z, Inoue T, Sakamoto A. Aprotinin in major orthopedic surgery: a systematic review of randomized controlled trials. Anesth Analg. 2005;101:1602–1607. doi: 10.1213/01.ANE.0000180767.50529.45. [DOI] [PubMed] [Google Scholar]
- 9.Findlay JY, Rettke SR, Ereth MH, Plevak DJ, Krom RA, Kufner RP. Aprotinin reduces red blood cell transfusion in orthotopic liver transplantation: a prospective, randomized, double-blind study. Liver Transpl. 2001;7:802–807. doi: 10.1053/jlts.2001.27086. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Huete L, Domenech P, Sabate A, Martinez-Brotons F, Jaurrieta E, Figueras J. The prophylactic effect of aprotinin on intraoperative bleeding in liver transplantation: a randomized clinical study. Hepatology. 1997;26:1143–1148. doi: 10.1002/hep.510260509. [DOI] [PubMed] [Google Scholar]
- 11.Fitzsimons MG, Peterfreund RA, Raines DE. Aprotinin administration and pulmonary thromboembolism during orthotopic liver transplantation: report of two cases. Anesth Analg. 2001;92:1418–1421. doi: 10.1097/00000539-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 12.O’Connor CJ, Roozeboom D, Brown R, Tuman KJ. Pulmonary thromboembolism during liver transplantation: possible association with antifibrinolytic drugs and novel treatment options. Anesth Analg. 2000;91:296–299. doi: 10.1097/00000539-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Porte RJ, Molenaar IQ, Begliomini B, Groenland TH, Januszkiewicz A, Lindgren L, Palareti G, Hermans J, Terpstra OT. Aprotinin and transfusion requirements in orthotopic liver transplantation: a multicentre randomised double-blind study. EMSALT Study Group. Lancet. 2000;355:1303–1309. doi: 10.1016/s0140-6736(00)02111-5. [DOI] [PubMed] [Google Scholar]
- 14.Marcel RJ, Stegall WC, Suit CT, Arnold JC, Vera RL, Ramsay MA, O’Donnell MB, Swygert TH, Hein HA, Whitten CW. Continuous small-dose aprotinin controls fibrinolysis during orthotopic liver transplantation. Anesth Analg. 1996;82:1122–1125. doi: 10.1097/00000539-199606000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Molenaar IQ, Veldman M, Begliomini B, Groenland HN, Januszkiewicz A, Lindgren L, Metselaar HJ, Terpstra OT, Porte RJ. Improved early graft survival in patients receiving aprotinin during orthotopic liver transplantation. Transplant Proc. 2001;33:1345–1346. doi: 10.1016/s0041-1345(00)02503-3. [DOI] [PubMed] [Google Scholar]
- 16.Dalmau A, Sabate A, Koo M, Bartolome C, Rafecas A, Figueras J, Jaurrieta E. The prophylactic use of tranexamic acid and aprotinin in orthotopic liver transplantation: a comparative study. Liver Transpl. 2004;10:279–284. doi: 10.1002/lt.20075. [DOI] [PubMed] [Google Scholar]
- 17.Llamas P, Cabrera R, Gomez-Arnau J, Fernandez MN. Hemostasis and blood requirements in orthotopic liver transplantation with and without high-dose aprotinin. Haematologica. 1998;83:338–346. [PubMed] [Google Scholar]
- 18.Weber T, Sendt W, Grube T, Scheele J. Coagulation profiles and intraoperative substitution requirements during elective piggyback liver transplantation with prophylactic antifibrinolytic therapy. Transpl Int. 2002;15:310–316. doi: 10.1007/s00147-002-0414-0. [DOI] [PubMed] [Google Scholar]
- 19.Ozier Y, Steib A, Ickx B, Nathan N, Derlon A, Guay J, De Moerloose P. Haemostatic disorders during liver transplantation. Eur J Anaesthesiol. 2001;18:208–218. doi: 10.1046/j.0265-0215.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- 20.Kang Y. Coagulopathies in hepatic disease. Liver Transpl. 2000;6:S72–S75. doi: 10.1002/lt.500060514. [DOI] [PubMed] [Google Scholar]
- 21.Porte RJ. Coagulation and fibrinolysis in orthotopic liver transplantation: current views and insights. Semin Thromb Hemost. 1993;19:191–196. doi: 10.1055/s-2007-994025. [DOI] [PubMed] [Google Scholar]
- 22.Porte RJ, Bontempo FA, Knot EA, Lewis JH, Kang YG, Starzl TE. Tissue-type-plasminogen-activator-associated fibrinolysis in orthotopic liver transplantation. Transplant Proc. 1989;21:3542. [PMC free article] [PubMed] [Google Scholar]
- 23.Dzik WH, Arkin CF, Jenkins RL, Stump DC. Fibrinolysis during liver transplantation in humans: role of tissue-type plasminogen activator. Blood. 1988;71:1090–1095. [PubMed] [Google Scholar]
- 24.Arnoux D, Boutiere B, Houvenaeghel M, Rousset-Rouviere A, Le Treut P, Sampol J. Intraoperative evolution of coagulation parameters and t-PA/PAI balance in orthotopic liver transplantation. Thromb Res. 1989;55:319–328. doi: 10.1016/0049-3848(89)90064-9. [DOI] [PubMed] [Google Scholar]
- 25.Backer CL, Kelle AM, Stewart RD, Suresh SC, Ali FN, Cohn RA, Seshadri R, Mavroudis C. Aprotinin is safe in pediatric patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2007;134:1421–1426; discussion 1426-1428. doi: 10.1016/j.jtcvs.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Zufferey P, Merquiol F, Laporte S, Decousus H, Mismetti P, Auboyer C, Samama CM, Molliex S. Do antifibrinolytics reduce allogeneic blood transfusion in orthopedic surgery? Anesthesiology. 2006;105:1034–1046. doi: 10.1097/00000542-200611000-00026. [DOI] [PubMed] [Google Scholar]
- 27.Molenaar IQ, Legnani C, Groenland TH, Palareti G, Begliomini B, Terpstra OT, Porte RJ. Aprotinin in orthotopic liver transplantation: evidence for a prohemostatic, but not a prothrombotic effect. Liver Transpl. 2001;7:896–903. doi: 10.1053/jlts.2001.27854. [DOI] [PubMed] [Google Scholar]
- 28.Dietrich W, Spath P, Ebell A, Richter JA. Prevalence of anaphylactic reactions to aprotinin: analysis of two hundred forty-eight reexposures to aprotinin in heart operations. J Thorac Cardiovasc Surg. 1997;113:194–201. doi: 10.1016/S0022-5223(97)70415-X. [DOI] [PubMed] [Google Scholar]
- 29.Beierlein W, Scheule AM, Ziemer G. Anaphylactic aprotinin reaction. Ann Thorac Surg. 2000;69:1298. doi: 10.1016/s0003-4975(00)01126-7. [DOI] [PubMed] [Google Scholar]
- 30.Molenaar IQ, Begliomini B, Grazi GL, Ringers J, Terpstra OT, Porte RJ. The effect of aprotinin on renal function in orthotopic liver transplantation. Transplantation. 2001;71:247–252. doi: 10.1097/00007890-200101270-00014. [DOI] [PubMed] [Google Scholar]
- 31.Ramsay MA, Randall HB, Burton EC. Intravascular thrombosis and thromboembolism during liver transplantation: antifibrinolytic therapy implicated? Liver Transpl. 2004;10:310–314. doi: 10.1002/lt.20064. [DOI] [PubMed] [Google Scholar]