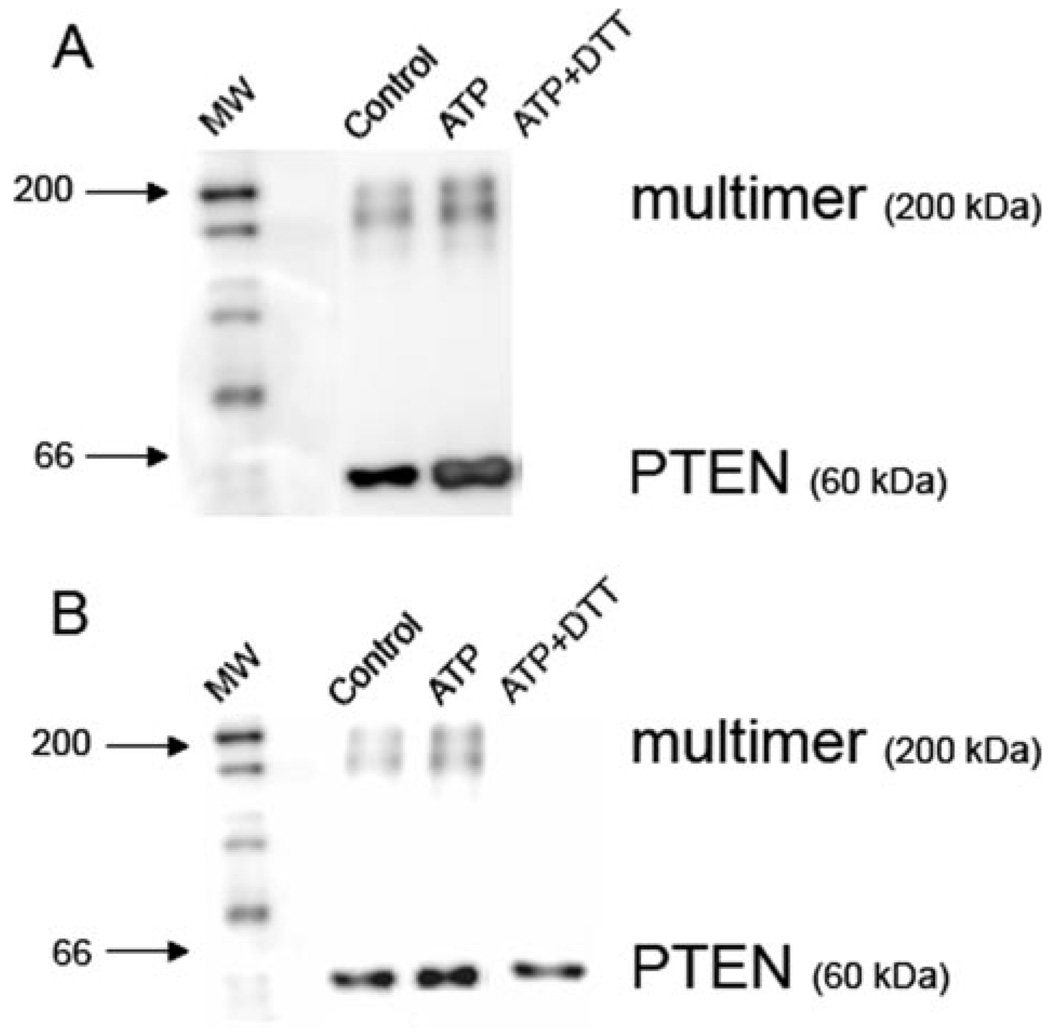
FIGURE 5. ATP-dependent ROS production induces glutathionylation of PTEN.
Macrophages were pretreated for 5 min with control buffer or 3 mm ATP for 1 min. Cells were lysed, and the proteins were immunoprecipitated overnight with PTEN-conjugated beads. A, the extent of glutathionylation was determined by Western blot, using an Ab recognizing GSH. The anti-GSH Ab revealed the presence of immunoprecipitated protein both as a monomer corresponding to the molecular mass of PTEN (60 kDa) or a larger complex (200 kDa). The level of glutathionylation of PTEN in both monomeric and multimeric forms increases after ATP stimulation of macrophages. Treatment of the immunoprecipitate with the reducing agent dithiothreitol before electrophoresis dissociates GSH from all proteins. B, the results were confirmed by reprobing the same membrane with an Ab recognizing total PTEN. The anti-PTEN Ab also revealed the presence of PTEN in monomers and a larger complex. Macrophage stimulation with ATP causes the amount of PTEN in the larger complex to increase slightly. Treatment with the reducing agent dithiothreitol causes PTEN to dissociate from the larger complex. The Western blots are representative of two experiments performed on separate days.