FIG. 3.
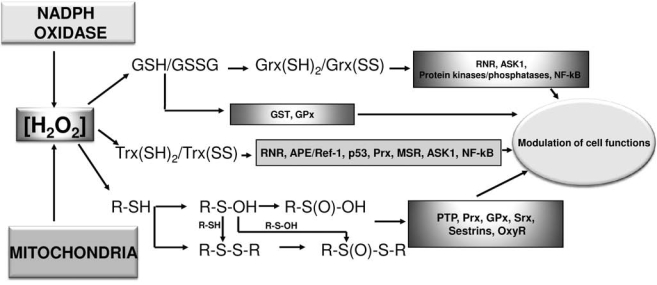
Signaling mediated by hydrogen peroxide. Hydrogen peroxide, produced by NADPH oxidase and/or mitochondria, can alter the redox state of glutathione (GSH/GSSG) and, in turn, of glutaredoxin [(Grx(SH)2/Grx(SS)] through the action of glutathione peroxidases. In a similar way, H2O2, after interaction with peroxiredoxins, modifies the redox balance of thioredoxin [Trx(SH)2/Trx(SS)]. Hydrogen peroxide can also interact with thiol groups of other enzymatic proteins leading to the formation of different oxidation states of sulfur. All these redox modifications of thiol redox state are mediated by sensitive proteins and lead to different consequences for the cell, going from proliferation to apoptosis.
