Abstract
Migraine is a debilitating condition characterized by recurrent severe head pain. Although mechanisms underlying a migraine attack remain controversial, one proposal is that inflammatory mediator (IM)–induced activation and sensitization of dural afferents contribute to the initiation of migraine pain. We and others have shown that the electrophysiological properties of afferents, both in the absence and the presence of IM, vary as a function of target of innervation. These differences may account for unique aspects of pain syndromes associated with specific body regions. Therefore the purpose of the present study was to test the hypothesis that the electrophysiological properties of dural afferents differ from those innervating the temporalis muscle (TM), a structure in close proximity to the dura but that is not associated with pain syndromes at all similar to migraine. Acutely dissociated retrograde labeled primary afferents innervating the dura and TM were examined with whole cell current-clamp recordings. Passive and active electrophysiological properties were determined before and after the application of IM: (in μM) prostaglandin E2 (1), bradykinin (10), and histamine (1). In the absence of IM, there were significant differences between the two populations, particularly with respect to the response to suprathreshold stimulation where dural afferents were more excitable than TM afferents. Importantly, although both populations of afferents were sensitized by IM, the pattern of passive and active electrophysiological changes associated with IM-induced sensitization of these two populations of afferents suggested that there were both similarities and marked differences between the two with respect to underlying mechanisms of sensitization. If the differences between dural and TM afferents are due to a differential pattern of ion channel expression rather than differences in the relative density/biophysical properties of the same ion channels, it may be possible to selectively treat migraine pain by targeting the distinct mechanisms underlying IM-induced sensitization of dural afferents.
INTRODUCTION
Migraine is a debilitating condition characterized by recurrent severe head pain, mechanical hypersensitivity, and other sensory dysfunctions (Burstein 2001). One hypothesis is that transient, recurrent release of inflammatory mediators (IMs) in the dura mater and resultant sensitization of dural sensory nerve endings are involved in the pain associated with a migraine. In support of this model, it has been demonstrated that levels of proinflammatory mediators, such as nitric oxide, calcitonin gene-related peptide (CGRP), neurokinin A, and prostaglandin E2 (PGE2) are increased at the onset of a migraine attack (Goadsby and Edvinsson 1993; Juhasz et al. 2005; Sarchielli et al. 2000). There is also evidence that exogenous application of IM onto the dura increases firing frequency, decreases the threshold of activation (Strassman et al. 1996), and increases the mechanosensitivity of dural afferents (Burstein 2001; Welch 2003).
In general, mechanisms of afferent sensitization may reflect changes in density, distribution, and biophysical properties of ion channels and receptors (McCleskey and Gold 1999). However, we and others have shown that there are not only differences between subpopulations of nociceptive afferents defined by target of innervation with respect to ion channel expression, but there are also differences in the mechanisms underlying the IM-induced sensitization of these subpopulations of nociceptive afferents. For example, primary afferents innervating cutaneous versus muscle tissues display differences in expression of purinergic receptor P2X3 (Ambalavanar et al. 2003, 2005) and in the relative density of different sodium channels (Oyelese and Kocsis 1996). There are also differences in ionic mechanisms underlying PGE2-induced sensitization of colonic and cutaneous afferents (Gold and Traub 2004).
There is evidence from both animal and human studies that dural afferents might have different electrophysiological properties as well. The dura mater has a dense accumulation of mast cells (Levy et al. 2007; Rozniecki et al. 1999) and a strikingly dense sympathetic innervation (Harriott and Gold 2008; Keller et al. 1989), both of which may contribute to potential differences in phenotypic properties of dural afferents. Additionally, there may be differences in the expression of neuropeptides and receptor ion channels in dural afferents. Recent studies suggest that a greater percentage of dural afferents express purinergic P2X receptors (Staikopoulos et al. 2007) and a greater proportion of dural afferents coexpress transient receptor potential vanilloid receptor 1 (TRPV1) with CGRP compared with the general trigeminal afferent population (Shimizu et al. 2007). Moreover, unlike stimulation of the muscle and skin, stimulation of the dura produces only the perception of pain in human subjects (Ray 1940). Last, although triptans, a class of antiinflammatory serotonin 1B/1D (5HT1B/1D) receptor agonists, are used to treat migraine pain, they are not generally used to treat other clinical pain conditions and, in a double-blind placebo-controlled trial, they were unable to relieve myofacial pain originating in the temporalis muscle (Dao et al. 1995). Taken together, these data suggest that mechanisms underlying sensitization of dural afferents may be different from those underlying sensitization of other afferent populations. Therefore to begin to test this suggestion, we have studied the electrophysiological properties of retrogradely labeled dural afferent somata in the presence and the absence of inflammatory mediators and compared results of this analysis with the properties of TM afferents studied under identical conditions.
METHODS
Animals
Adult female Sprague–Dawley rats (Harlan, Indianapolis, IN) weighing between 175 and 280 g were used for all experiments. Female rats were chosen because migraine is significantly more common in women than in men. However, we did not test sex-related differences in afferent activity in this study. Rats were housed two per cage in the University of Pittsburgh animal facility on a 12:12 light:dark schedule with food and water freely available. Prior to all procedures, animals were deeply anesthetized with an intraperitoneal injection (1 ml/kg) of rat cocktail containing ketamine (55 mg/kg), xylazine (5.5 mg/kg), and acepromazine (1.1 mg/kg). Experiments were approved by the University of Pittsburgh Institutional Animal Care and Use Committee and performed in accordance with National Institutes of Health guidelines for the use of laboratory animals in research.
Identification of dural and temporalis muscle (TM) afferents
Two populations of neurons were identified: 1) afferents innervating the dura and 2) afferents innervating the temporalis muscle. These subpopulations were identified as previously described (Harriott and Gold 2008). Briefly, dural afferents were labeled with the retrograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, Invitrogen, Carlsbad, CA) applied to a section of the dura surrounding the superior sagittal sinus bilaterally. The skin overlying the cranium was shaved and disinfected with betadine solution. A longitudinal midsagittal incision was made into the skin exposing the cranium. To expose the meninges, a square portion of the cranium overlying the superior sagittal sinus was removed, leaving the underlying meninges intact and exposed for the application of retrograde tracer. A single droplet (2–4 μl) of DiI (170 mg/ml in DMSO and diluted 1:10 in saline) was applied onto the exposed meningeal tissues using a 30-gauge needle attached to a Hamilton syringe. A rubber dam (Salama et al. 1994) was placed on the exposed dura to prevent leakage of tracer and secured to the cranium with superglue adhesive. An acrylic cap was used to replace the removed cranium and the incision was closed with silk sutures. TM afferents were labeled with DiI injected into the body of the muscle. A single incision was made in the skin in the area of the TM and 1–2 μl of DiI was injected into each of five locations (total volume 5–10 μl). The needle was held in place for 10 s after each injection to prevent the leakage of tracer when the needle was withdrawn. The incision was closed with sutures. Immediately postoperatively, animals received a single intramuscular injection of penicillin G (10,000 units/kg) and a single injection of buprenorphine (0.03 mg/kg) to minimize infection and discomfort.
Tissue preparation
Ten days following DiI application, trigeminal ganglia were removed, enzymatically treated, and mechanically dissociated as previously described (Harriott et al. 2006). Acutely dissociated cells were plated on laminin/ornithine-coated glass coverslips. Baseline and IM-induced changes in excitability were measured 2–8 h after cells were plated.
Current-clamp electrophysiology
All whole cell patch-clamp recordings were performed with a HEKA EPC10 amplifier (HEKA Elektronik, Lambrecht/Rhineland-Pfalz, Germany). Data were low-pass filtered at 5–10 kHz with a four-pole Bessel filter and digitally sampled at 25–100 kHz. For current-clamp experiments borosilicate glass electrodes were 1–4 MΩ when filled with (in mM) K-methanesulfonate 110, KCl 30, NaCl 5, CaCl2 1, MgCl2 2, HEPES 10, EGTA 11, Mg-ATP 2, and Li-GTP 1 (pH 7.2, adjusted with Tris-base; 310 mOsm, adjusted with sucrose). Bath solutions contained (in mM) KCl 3, NaCl 130, CaCl2 2.5, MgCl2 0.6, HEPES 10, glucose 10, and either vehicle (0.01% ETOH and 0.1% acetic acid) or IM (in μM) bradykinin 10, histamine 1, and prostaglandin E2 1 (pH 7.4, adjusted with Tris-base; 325 mOsm, adjusted with sucrose). All salts were obtained from Sigma–Aldrich (St. Louis, MO), unless otherwise indicated.
Changes in excitability were recorded in the presence and the absence of IM. Four parameters were used to assess afferent excitability: 1) action potential (AP) threshold, defined as the greatest membrane depolarization before AP generation in response to 750-ms depolarizing current injection; 2) rheobase, defined as the least amount of depolarizing current needed to evoke a single AP; 3) response to suprathreshold stimulation, defined by the number of APs evoked by depolarizing current injection 1.5-, 2-, 2.5-, and 3-fold rheobase; and 4) spontaneous activity. Baseline excitability (mean ± SD for each parameter) was determined with the application of at least three stimulation protocols or 30-s observation intervals (for spontaneous activity) prior to the application of inflammatory mediators. A neuron was considered sensitized if the application of inflammatory mediators resulted in a hyperpolarization of AP threshold, decrease in rheobase, an increase in the response to suprathreshold stimulation, and/or an increase in spontaneous activity >2SDs from the baseline mean. We also used ramp-current injections (1 nA over 1 s) to monitor changes in current threshold, voltage threshold, and accommodation with a single stimulus, enabling rapid assessment of baseline properties.
To begin to assess the relative contribution of specific ion channels on baseline differences and IM-induced changes in excitability, passive and active properties of dural and TM afferents were analyzed. Passive properties included cell capacitance (CpF), resting membrane potential (Em), and input resistance (Rin). For comparisons between tissues, these were determined at the beginning of each recording. After gaining whole cell access, membrane capacitance was determined with amplifier circuitry. For between-group comparisons of Rin, Rin was measured at the beginning of the recording using the average of four current traces evoked by 10-ms voltage steps to −90 mV from a holding potential of −70 mV. Within 10 s of establishing whole cell access, the amplifier was then switched to current-clamp mode for the assessment of spontaneous activity. Em was determined during the first assessment of spontaneous activity. To assess the impact of IM on Rin, Rin was assessed with five 750-ms hyperpolarizing current injections (2–5 pA) from Em immediately before and 90 s after the application of IM. Active electrophysiological properties were assessed with an AP evoked with a 4-ms depolarizing current pulse. These included: AP duration at 0 mV, magnitude of AP overshoot, magnitude of the afterhyperpolarization (AHP), AHP decay (τAHP), and rates of AP rise and fall. The magnitude of the overshoot was measured from 0 mV. The magnitude of the AHP was measured from the Em. Decay of the AHP was estimated by fitting the decay phase of the AHP with a single exponential function. Rates of AP rise and fall were determined from the first derivative of the AP waveform. Data analysis was performed with PulseFit (HEKA), Sigma plot, and Sigma Stat software (Systat Software, Richmond, CA).
Statistical analysis
Differences in baseline rheobase and threshold between dural and TM afferents were compared with a Student's t-test if data were normally distributed and a Mann–Whitney rank-sum test if data were not normally distributed. Changes in excitability and passive and active electrophysiological properties, before and after inflammatory mediators, were determined with a paired t-test. Data are expressed as the mean ± SE.
RESULTS
Dural versus TM afferents: differences in baseline electrophysiological properties
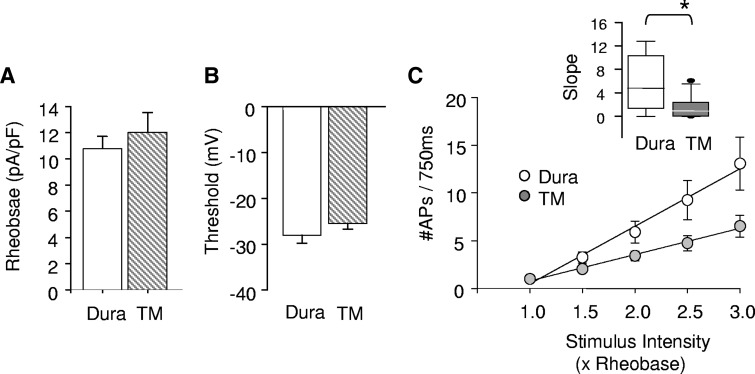
We examined 65 dural and 48 TM afferents from 22 female Sprague–Dawley rats. Neither dural (n = 65) nor TM (n = 48) afferents displayed spontaneous activity in the absence of inflammatory mediators. There were also no significant differences between these two subpopulations of afferents with respect to rheobase or AP threshold (Fig. 1, A and B). However, the two did differ in their response to suprathreshold stimulation (Fig. 1C), although there was a linear increase in the number of APs evoked in response to stimuli between 1.5- and 3-fold rheobase in both populations, the slope of this relationship was significantly (P < 0.01, Fig. 1C, inset) greater for dural afferents (5.92 ± 1.3 AP/fold increase in rheobase) than that for TM afferents (1.65 ± 0.4).
FIG. 1.
Dural (n = 26) and temporalis muscle (TM; n = 25) afferents show differences in baseline excitability. There were no significant differences between dural and TM afferents with respect to rheobase (A) or action potential (AP) threshold (B). However, dural afferents differed from TM afferents in response to suprathreshold stimulation with post hoc analysis revealing significantly greater APs at stimuli that were 2.5- and 3-fold rheobase in dural compared with TM afferents (C). Inset: when stimulus response functions were fit with a linear function, dural afferents had a significantly steeper slope compared with that of TM afferents. Slope plotted as median 5th/95th percentile. * Indicates significant differences where P < 0.05.
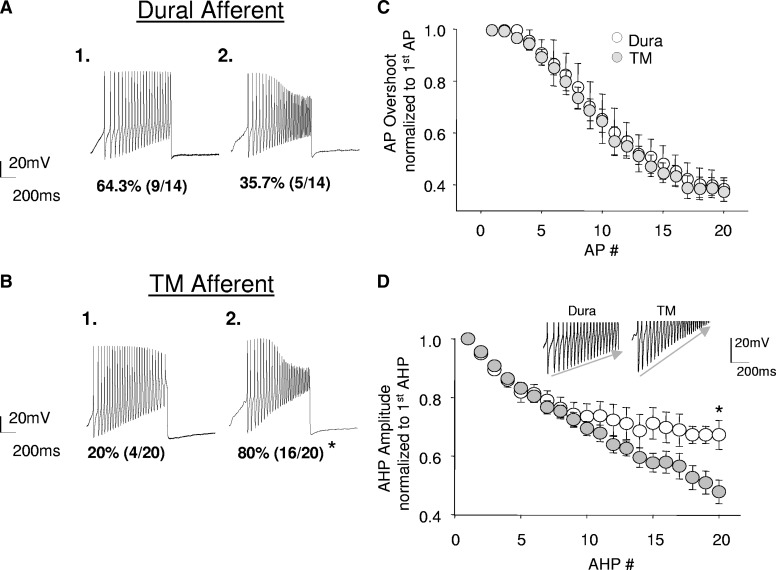
It also became readily apparent that there were at least two subpopulations of both dural and TM afferents detectable in the baseline response to ramp-current injection. In one population, there was little, if any, decrement in the AP overshoot throughout the sustained AP barrage (Fig. 2 A1). In the other population, the AP overshoot was dramatically reduced by the end of the AP barrage (Fig. 2A2). Interestingly, there was a significant difference between dural and TM afferents with respect to the proportion of neurons with and without use-dependent attenuation of the AP overshoot (Fig. 2, A and B). In neurons that exhibited a use-dependent decrease in AP overshoot, the magnitude (Fig. 2C) of the decrease was similar between the two populations. The use-dependent decrease in the AP overshoot did not appear to be directly related to the magnitude of the AHP because the AHP decreased during sustained activity in neurons with and without use-dependent decreases in AP overshoot. However, the use-dependent diminution in AHP amplitude was greater in TM than that in dural afferents (Fig. 2D).
FIG. 2.
The distribution of use-dependent inhibition of AP overshoot and use-dependent inhibition of afterhyperpolarization (AHP) magnitude differ between dural (n = 14) and TM afferents (n = 20). Subpopulations of both dural (A) and TM (B) afferents displayed use-dependent decreases in AP overshoot. A significantly greater proportion of TM afferents (16/20) displayed this property compared with dural (5/14) afferents. There was no difference in the magnitude of use-dependent inhibition in the population of dural (n = 5) and TM (n = 16) afferents that did display this property (C). Both dural and TM afferents also displayed use-dependent inhibition in AHP amplitude with successive APs. However, TM (n = 20) afferents had a significantly greater reduction compared with that of dural (n = 14) afferents (D). Inset: representative decay of AHP magnitude in dural and TM afferents following repetitive firing. * Indicates significant differences, where P < 0.05, χ2 test, Student's t-test.
To identify putative ion channels that may underlie these baseline differences in response to suprathreshold stimulation, we examined both passive and active electrophysiological properties. There were no differences in median cell capacitance between dural (23.9 pF, n = 26) and TM (24.0 pF, n = 25) afferents and no differences in the resting membrane potential; however, dural afferents had a significantly lower baseline input resistance compared with that of TM afferents (Table 1). There were also clear differences between dural and TM afferents with respect to active electrophysiological properties: the AP overshoot was significantly greater in dural afferents compared with that in TM afferents, the afterhyperpolarization (AHP) amplitude was significantly smaller, and the AHP decay rate was significantly slower in dural than that in TM afferents (Table 1).
TABLE 1.
Differences in baseline active electrophysiological properties between dural (n = 26) and temporalis muscle (TM) afferents (n = 25)
| Em, mV | Rin, MΩ | Cm, pF | AP Duration, ms | AP Overshoot, mV | AHP Magnitude, mV | τAHP, ms | Rate Rise, dV/dt | Rate Fall, dV/dt | |
|---|---|---|---|---|---|---|---|---|---|
| Dura | −71.3 ± 1.6 | 473 ± 57.3 | 23.9 (19.2 to 31.5) | 2.6 ± 0.3 | 45.9 ± 1.2 | −9.4 ± 0.9 | 95.0 ± 13.0 | 124.5 ± 17.4 | −66.1 ± 4.0 |
| TM | −68.3 ± 1.7 | 786 ± 79.0‡ | 24.7 (19.6 to 26.1) | 3.1 ± 0.3 | 41.7 ± 1.5‡ | −14.4 ± 1.1‡ | 56.3 ± 9.1‡ | 116.7 ± 13.0 | −56.1 ± 8.0 |
Values are means ± SE, except for Cm, which is expressed as median (25th to 75th percentile). Em, resting membrane potential; Rin, input resistance resistance; Cm, membrane capacitance; AP, action potential; AHP, after hyperpolarization; τAHP, time constant for the decay of the AHP; Rate Rise, the maximal rate of rise of the AP; Rate Fall, the maximal rate of fall of the AP.
Indicates significant differences, where P < 0.05, Student's t-test.
Dural versus TM afferents: differences in the response to inflammatory mediators
To examine the target of innervation differences in response to IM, excitability was assessed before and after bath application of an inflammatory “soup,” consisting of PGE2 (1 μM), bradykinin (10 μM), and histamine (1 μM). Previous studies indicate that similar IM combinations are sufficient to sensitize trigeminovascular afferents in vivo and increase the responsiveness of brain stem neurons to mechanical stimulation of the dura and face (Burstein et al. 1998). All dural afferents (19/19) were sensitized by IM application (as defined in methods). In contrast, 6/21 TM afferents studied did not display a change in any of the parameters for excitability and were considered unresponsive. The difference in the proportion of dural and TM afferents that were sensitized by IM was significant (P < 0.05, Fisher exact test). In both afferent populations, IM-induced sensitization was not associated with the emergence of spontaneous activity. IM-induced sensitization of all dural afferents (19/19) was associated with an increase in excitability, as determined by the remaining three measures used: a decrease in rheobase (P < 0.01), a decrease in AP threshold (P < 0.01), and an increase in the response to suprathreshold stimuli (P < 0.01; Fig. 3). In contrast, IM-induced sensitization of TM afferents was associated with a decrease in rheobase (P < 0.05) and an increase in the response to suprathreshold stimuli (P < 0.05), but no change in AP threshold (P > 0.05; Fig. 3).
FIG. 3.
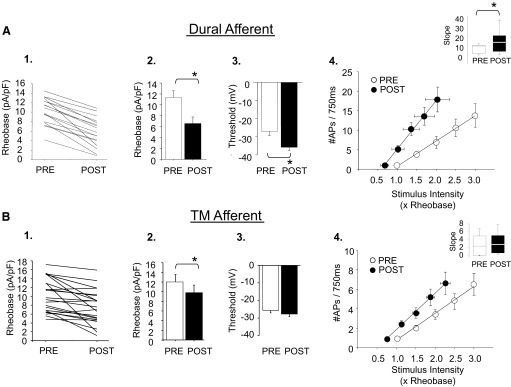
Inflammatory mediators (IMs) increased excitability of dural (n = 19) and TM afferents (n = 21). IM decreased rheobase 2SDs beyond the mean baseline response in 19/19 dural afferents studied (individual cells: A1; population: A2). IM also hyperpolarized the AP threshold of dural afferents (A3) and left-shifted the stimulus response function (A4). Inset: the slope of the stimulus response function was significantly larger in dural afferents after IM. In contrast to dural afferents, IM application did not decrease rheobase 2SDs beyond in the mean baseline response in 9/21 TM afferents (individual cells: B1): 6 of those 9 did not show differences in any parameter for excitability and were considered unresponsive; 15/21 TM afferents responded to IM with a significant decrease in rheobase (B2) and a left-shifted stimulus response function (B4). Unlike dural afferents, there was no effect of IM on the AP threshold of TM afferents (B3). Slope plotted as median 5th/95th percentile. * Indicates significant differences, where P < 0.05, paired t-test.
IM-induced changes in passive and active electrophysiological properties
To identify putative ion channels that may underlie IM-induced sensitization of dural and TM afferents, we assessed the impact of IM on passive and active electrophysiological properties.
IM-induced sensitization of dural and TM afferents was associated with a distinct pattern of changes in passive electrophysiological properties. IM depolarized the resting membrane potential of dural afferents. This change in membrane potential was significant (P < 0.01) and was accompanied by a significant (P < 0.01) decrease in input resistance (Table 2). In contrast, IM produced the opposite effect (P < 0.05) on the input resistance of TM afferents with no change in the resting membrane potential (Table 2).
TABLE 2.
Distinct ionic mechanisms underlie IM-induced sensitization of dural (n = 19) and TM afferents (n = 15)
| Em, mV | Rin, MΩ | AP Duration, ms | AP Overshoot, mV | AHP Magnitude, mV | τAHP, ms | Rate Rise, dV/dt | Rate Fall, dV/dt | |
|---|---|---|---|---|---|---|---|---|
| Dura | ||||||||
| Pre-IM | −71.50 ± 6.4 | 500.20 ± 77.1 | 3.37 ± 0.40 | 45.02 ± 1.7 | −11.51 ± 2.7 | 92.03 ± 14.2 | 124.54 ± 17.4 | −66.14 ± 4.0 |
| Post-IM | −62.00 ± 5.4‡ | 293.11 ± 62.9‡ | 2.66 ± 0.50‡ | 52.93 ± 1.7‡ | −18.33 ± 1.3‡ | 28.53 ± 7.3‡ | 132.20 ± 18.1 | −62.27 ± 2.5 |
| Post-IM§ | −71.00 ± 2.7 | 286.40 ± 53.8‡ | 3.42 ± 0.50 | 50.74 ± 1.8‡ | −13.02 ± 2.4 | 88.09 ± 11.7 | 143.72 ± 11.9‡ | −61.70 ± 2.8 |
| TM | ||||||||
| Pre-IM | −67.33 ± 8.8 | 1,028.88 ± 152.7 | 3.00 ± 0.62 | 40.53 ± 1.8 | −11.91 ± 1.1 | 70.60 ± 15.2 | 119.92 ± 26.4 | −65.00 ± 4.7 |
| Post-IM | −65.72 ± 7.1 | 1,228.80 ± 140.9* | 3.06 ± 0.49 | 49.33 ± 1.6* | −12.23 ± 1.3 | 69.04 ± 16.1 | 139.23 ± 24.5* | −61.88 ± 6.2 |
Values are means ± SE. Pre-IM, values obtained prior to the application of inflammatory mediators; Post-IM, values obtained following the application of inflammatory mediators. All other abbreviations are used as in Table 1
Indicates significant differences before and after IM application to dural afferents, where P < 0.05, paired t-test.
Indicates significant differences before and after IM application to TM afferents, where P < 0.05, paired t-test.
Indicates that in dural afferents active electrophysiological properties were recorded while preventing the IM-induced change in membrane potential with DC current injection.
There were a number of active electrophysiological properties in dural afferents that were significantly changed following IM application. These included AP duration and overshoot as well as the AHP magnitude (Table 2). To determine the extent to which the complex changes in the AP waveform following IM were secondary to IM-induced membrane depolarization, changes in excitability and AP waveform were examined in a group (n = 17) of dural afferents where the baseline Em was maintained with DC injection. Under these conditions dural afferents were still sensitized following IM application, as indicated by a significant (P < 0.01) reduction in rheobase (11.74 ± 1.7 pre-IM; 8.37 ± 1.2 post-IM), hyperpolarization of AP threshold (−25.23 ± 2.4 pre-IM; −31.30 ± 1.6 post-IM), and an increase in the slope of the stimulus response function (4.45 ± 1.1 pre-IM; 8.94 ± 2.1 post-IM), although the magnitude of these changes were in general smaller than those seen in the presence of depolarization. In the absence of membrane depolarization, IM significantly increased the AP overshoot and rate of rise of dural afferents. IM also increased the AP overshoot of TM afferents and increased the rate of rise of the AP (Table 2).
DISCUSSION
We tested the hypothesis that dural afferents have different electrophysiological properties in both the absence and presence of IM compared with afferents innervating myofacial tissues. Consistent with this hypothesis, our data suggest that there are baseline differences in excitability between dural and TM afferents. Moreover, IM appears to selectively activate a depolarizing conductance in dural afferents, whereas in TM afferents there appears to be a decrease in conductance that was not associated with a change in the resting membrane potential.
Selection of afferent populations
Several nonvisceral pain conditions, such as migraine, are characterized by pain restricted to specific peripheral tissues, including joint pain and myalgia. Temporomandibular disorders (TMDs), another set of debilitating conditions, are characterized by pain restricted to the masticatory muscles including the temporalis muscle and/or temporomandibular joint. Similar to migraine, TMD may involve peripheral tissue inflammation (Alstergren and Kopp 2000; Kopp 2001). Since both dural and TM afferents have cell bodies located in the trigeminal ganglia and are involved in nociceptive signaling in the presence of inflammation, TM afferents were used as the comparison population in this study.
Baseline differences: the contribution of labeling and sample bias
It is important to consider the possibility that the observed differences between dural and TM afferents reflects our labeling procedures or sampling bias. For example, it is possible that DiI administration results in the initiation of a transient inflammatory response and there is evidence that craniotomy alone may produce mast cell degranulation and, consequently, sensitization of dural afferents (Levy et al. 2007). However, we were unable to detect evidence of persistent inflammation in the TM, 10 days following DiI application (data not shown). Furthermore, although not systematically analyzed, we observed intact mast cells with toluidine blue staining of dural tissues 10 days after DiI application (Supplemental Fig. S1).1 Additionally, we did not observe any spontaneous activity in dural afferents, in contrast to the emergence of spontaneous activity observed in neurons innervating sites of persistent inflammation (Flake et al. 2005), indicating that DiI application probably did not induce a persistent inflammation in these animals. Thus although it is impossible to avoid perturbing the “system” to study it, we suggest that the labeling procedure, per se, is unlikely to account for the differences observed between dural and TM afferents.
There is also a possibility that the differences in ionic mechanisms responsible for baseline excitability and/or IM-induced sensitization reflect a systematic bias in our sampling of one or the other afferent populations, particularly in light of the functional heterogeneity of sensory neurons. We (Gold et al. 1996a,b) and others (Ambalavanar et al. 2003; Djouhri et al. 2003; Staikopoulos et al. 2007; Tu et al. 2004) have demonstrated that some of this heterogeneity is manifest as a function of cell body diameter, where the expression of ion channels and other proteins in small-diameter primary afferents is distinct from that in medium- or large-diameter neurons. In an effort to diminish cell-size bias between afferent populations, we recorded from the first labeled dural or TM afferent found on each coverslip. Accordingly, the distribution of cell capacitance from the dural afferent population sampled overlapped with that of TM afferents. Additionally, unlike the dorsal root ganglion (DRG), cell bodies giving rise to the most rapidly conducting fibers that function in proprioception are not located in the TG but rather in the mesencephalic nucleus, limiting some of the possibility of heterogeneity bias.
Underlying mechanisms of baseline differences in excitability
In support of the suggestion that observed differences between dural and TM afferents may reflect intrinsic differences in the expression, relative number, and/or function of ion channels, there are now a number of electrophysiological studies demonstrating differences in excitability (Dang et al. 2004; Harriott et al. 2006; Moore et al. 2002; Stewart et al. 2003; Yoshimura and de Groat 1999) as well as anatomical studies demonstrating differences in ion channel distribution (Ambalavanar et al. 2005; Shimizu et al. 2007; Staikopoulos et al. 2007) between subpopulations of afferents defined by target of innervation. The differences between dural and TM afferents with respect to passive and active electrophysiological properties were 1) the input resistance, 2) the magnitude and decay of the AHP, 3) the magnitude of use-dependent decay in the AHP amplitude, 4) the magnitude of the AP overshoot, and 5) the relative distribution of neurons exhibiting a use-dependent decay in the AP overshoot. Baseline differences in input resistance between dural and TM afferents suggest that there are more channels open at rest in dural compared with TM afferents. Despite the lower input resistance, there were no differences in resting membrane potential or rheobase.
We suggest that differences in the magnitude and decay of the AHP and use-dependent decay of the AHP are likely to reflect differences in the expression or biophysical properties of voltage-gated or Ca2+-dependent K+ channels. In sensory neurons, there is a diversity of K+ channels expressed in subpopulations of sensory afferents (Gold et al. 1996a). Since we did not observe differences in AP threshold or AP repolarization, the differences in AHP amplitude and decay are probably not due to a large-conductance Ca2+-dependent K+ channel (Scholz et al. 1998) or a delayed-rectifier type of K+ channel (Werz and MacDonald 1983), given the apparent role for these channels in AP repolarization in sensory neurons. Instead these differences in the AHP amplitude and decay are more consistent with a difference in the relative density or biophysical properties of a low-threshold K+ channel largely inactivated at resting membrane potentials (Cardenas et al. 1995; Gold et al. 1996a), a hyperpolarization-activated channel subject to use-dependent inhibition (Cardenas et al. 1995), and/or an intermediate-conductance Ca2+-dependent K+ channel (Fowler et al. 1985).
We suggest that the differences in magnitude of the AP overshoot possibly reflect a greater number of voltage-gated Na+ channels in dural afferents. Furthermore, inactivation of the tetrodotoxin-resistant (TTX-R) Na+ channel Nav1.8 likely accounts for differences in the relative distribution of dural and TM afferents exhibiting a use-dependent decay in the AP overshoot. Data from previous studies suggest that the TTX-R Na+ channel underlies the AP upstroke and overshoot in the majority of nociceptive afferents (Renganathan et al. 2001) and our own preliminary results suggest that from the average resting membrane potential of dural and TM afferents, this channel underlies 88.3 and 85.1% of the total Na+ current density, respectively. Previous data also suggest that subpopulations of nociceptive afferents can be identified based on the presence of a use-dependent decay in the AP overshoot (Choi et al. 2007; Yamane et al. 2007). This phenomenon appears to reflect the development of slow inactivation of Nav1.8 channels, which may develop much more rapidly in some sensory neurons than in others. Given that a single gene encoding Nav1.8 has been identified, differences in the rate at which channels enter into a slowly inactivated state is likely to reflect posttranslational modification of this channel. Interestingly, although previous studies suggest that subpopulations of sensory neurons with slowly inactivating Nav1.8 channels can be defined by the presence of IB4 binding or capsaicin sensitivity (Gold 2008), results of the present study suggest that this property is also differentially distributed among subpopulations defined by target of innervation.
Underlying mechanisms of IM-induced sensitization
IM increased excitability in both dural and TM afferents, although there appears to be greater heterogeneity in TM compared with dural afferents; 100% of dural afferents were sensitized by IM in contrast to 78% of TM afferents. The uniformity in the dural afferent response is consistent with the notion that dural afferents are responsible for processing only nociceptive signals and suggests that a significant minority of TM afferents subserve nonnociceptive functions and/or is responsive to a unique mix of IM. Importantly, however, differences in the response rate are not responsible for differences in the pattern of IM-induced changes in excitability because dural and TM afferents were still distinguishable with nonresponders eliminated from analysis.
Application of IM produced an increase in AP amplitude and rate of rise, suggestive of increases in Na+ currents in both dural and TM afferents. Data from a number of studies illustrate the important role of Na+ channels in the IM-induced sensitization of sensory neurons where a variety of IMs have been shown to increase TTX-R INa as well as TTX-S INa (at least in some preparations; Amir et al. 2006). More recent evidence supports a role for persistent Na+ currents (i.e., those carried by Nav1.9) in the actions of inflammatory mediators (Maingret et al. 2008). In previous studies, the IM-induced increase in current appears to reflect, at least in part, direct phosphorylation of the Na+-channel alpha subunit (Fitzgerald et al. 1999). It has also been shown that PGE2 can reduce K+ current in sensory neurons (Evans et al. 1999). However, the lack of IM-induced effects on the AHP or AP duration of either dural or TM afferents argues against an inhibition of voltage-gated K+.
One of the most interesting observations of this study is that IM depolarized the resting membrane potential of dural but not TM afferents. That this depolarization was accompanied by a decrease in input resistance argues for the selective activation of a depolarizing current. We were able to demonstrate that an IM-induced increase in excitability was still detectable when the depolarization was reversed with DC injection. Although a depolarization-induced inactivation of ion channels can result in net inhibition, the fact that dural afferents were even more excitable when depolarized suggests that the depolarization contributes to the IM-induced sensitization of dural afferents. To further test this conclusion, the resting membrane potential of a group of dural afferents was depolarized in the absence of IM with DC injection. Importantly, depolarization alone was able to increase excitability (decrease rheobase, hyperpolarize threshold, and left-shift the SRF; Supplemental Fig. S2).
Although not assessed in the present study, there are several ion channels that may contribute to the IM-induced depolarization of dural afferents. One of these channels is TRPV1, given previous data indicating that bradykinin can increase resting whole cell and single-channel inward current in DRG neurons via increases in TRPV1 activity (Shin et al. 2002; Sugiura et al. 2002). There is also evidence that prostaglandin can depolarize the voltage dependence of activation of hyperpolarization-activated, cyclic nucleotide-gated cation (HCN) channels (Ingram and Williams 1996). Importantly, depolarizing shifts in the voltage dependence of activation of these channels may produce the same pattern of changes in electrophysiological properties observed with inflammatory mediator application to dural afferents. It is also possible that activation of Nav1.9 may contribute to the reduction in input resistance, membrane depolarization, and increases in excitability (Baker et al. 2003).
Summary and Conclusions
In summary, we now show that dural afferents have very different baseline electrophysiological properties and IM-induced changes in excitability compared with those of facial muscle afferents. The ionic mechanisms underlying these differences in electrophysiological properties raise the possibility that we may be able to develop novel therapeutic agents that selectively modulate dural afferent excitability. These agents could prove useful for the treatment of migraine pain. Further experiments are needed to determine which ion channels contribute to the IM-induced depolarization of dural afferents and whether these channels could be targets for future drug therapy.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-059153 to A. M. Harriott and NS-41384 to M. S. Gold.
Supplementary Material
Acknowledgments
We thank Dr. Daniel Weinreich for helpful discussions during preparation of this manuscript.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Alstergren and Kopp 2000.Alstergren P, Kopp S. Prostaglandin E2 in temporomandibular joint synovial fluid and its relation to pain and inflammatory disorders. J Oral Maxillofac Surg 58: 180–186; discussion 186–188, 2000. [DOI] [PubMed] [Google Scholar]
- Ambalavanar et al. 2005.Ambalavanar R, Moritani M, Dessem D. Trigeminal P2X3 receptor expression differs from dorsal root ganglion and is modulated by deep tissue inflammation. Pain 117: 280–291, 2005. [DOI] [PubMed] [Google Scholar]
- Ambalavanar et al. 2003.Ambalavanar R, Moritani M, Haines A, Hilton T, Dessem D. Chemical phenotypes of muscle and cutaneous afferent neurons in the rat trigeminal ganglion. J Comp Neurol 460: 167–179, 2003. [DOI] [PubMed] [Google Scholar]
- Amir et al. 2006.Amir R, Argoff CE, Bennett GJ, Cummins TR, Durieux ME, Gerner P, Gold MS, Porreca F, Strichartz GR. The role of sodium channels in chronic inflammatory and neuropathic pain. J Pain 7: S1–S29, 2006. [DOI] [PubMed] [Google Scholar]
- Baker et al. 2003.Baker MD, Chandra SY, Ding Y, Waxman SG, Wood JN. GTP-induced tetrodotoxin-resistant Na+ current regulates excitability in mouse and rat small diameter sensory neurones. J Physiol 548: 373–382, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein 2001.Burstein R Deconstructing migraine headache into peripheral and central sensitization. Pain 89: 107–110, 2001. [DOI] [PubMed] [Google Scholar]
- Burstein et al. 1998.Burstein R, Yamamura H, Malick A, Strassman AM. Chemical stimulation of the intracranial dura induces enhanced responses to facial stimulation in brain stem trigeminal neurons. J Neurophysiol 79: 964–982, 1998. [DOI] [PubMed] [Google Scholar]
- Cardenas et al. 1995.Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol 74: 1870–1879, 1995. [DOI] [PubMed] [Google Scholar]
- Choi et al. 2007.Choi JS, Dib-Hajj SD, Waxman SG. Differential slow inactivation and use-dependent inhibition of Nav1.8 channels contribute to distinct firing properties in IB4+ and IB4− DRG neurons. J Neurophysiol 97: 1258–1265, 2007. [DOI] [PubMed] [Google Scholar]
- Dang et al. 2004.Dang K, Bielefeldt K, Gebhart GF. Gastric ulcers reduce A-type potassium currents in rat gastric sensory ganglion neurons. Am J Physiol Gastrointest Liver Physiol 286: G573–G579, 2004. [DOI] [PubMed] [Google Scholar]
- Dao et al. 1995.Dao TT, Lund JP, Remillard G, Lavigne GJ. Is myofascial pain of the temporal muscles relieved by oral sumatriptan? A cross-over pilot study. Pain 62: 241–244, 1995. [DOI] [PubMed] [Google Scholar]
- Djouhri et al. 2003.Djouhri L, Fang X, Okuse K, Wood JN, Berry CM, Lawson SN. The TTX-resistant sodium channel Nav1.8 (SNS/PN3): expression and correlation with membrane properties in rat nociceptive primary afferent neurons. J Physiol 550: 739–752, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans et al. 1999.Evans AR, Vasko MR, Nicol GD. The cAMP transduction cascade mediates the PGE2-induced inhibition of potassium currents in rat sensory neurones. J Physiol 516: 163–178, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald et al. 1999.Fitzgerald EM, Okuse K, Wood JN, Dolphin AC, Moss SJ. cAMP-dependent phosphorylation of the tetrodotoxin-resistant voltage-dependent sodium channel SNS. J Physiol 516: 433–446, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake et al. 2005.Flake NM, Bonebreak DB, Gold MS. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol 93: 1585–1597, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler et al. 1985.Fowler JC, Greene R, Weinreich D. Two calcium-sensitive spike after-hyperpolarizations in visceral sensory neurones of the rabbit. J Physiol 365: 59–75, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goadsby and Edvinsson 1993.Goadsby PJ, Edvinsson L. The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann Neurol 33: 48–56, 1993. [DOI] [PubMed] [Google Scholar]
- Gold 2008.Gold MS Na(+) channel blockers for the treatment of pain: context is everything, almost. Exp Neurol 210: 1–6, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold et al. 1996a.Gold MS, Shuster MJ, Levine JD. Characterization of six voltage-gated K+ currents in adult rat sensory neurons. J Neurophysiol 75: 2629–2646, 1996a. [DOI] [PubMed] [Google Scholar]
- Gold et al. 1996b.Gold MS, Shuster MJ, Levine JD. Role of a Ca(2+)-dependent slow afterhyperpolarization in prostaglandin E2-induced sensitization of cultured rat sensory neurons. Neurosci Lett 205: 161–164, 1996b. [DOI] [PubMed] [Google Scholar]
- Gold and Traub 2004.Gold MS, Traub RJ. Cutaneous and colonic rat DRG neurons differ with respect to both baseline and PGE2-induced changes in passive and active electrophysiological properties. J Neurophysiol 91: 2524–2531, 2004. [DOI] [PubMed] [Google Scholar]
- Harriott et al. 2006.Harriott AM, Dessem D, Gold MS. Inflammation increases the excitability of masseter muscle afferents. Neuroscience 141: 433–442, 2006. [DOI] [PubMed] [Google Scholar]
- Harriott and Gold 2008.Harriott AM, Gold MS. Serotonin type 1D receptors (5HTR) are differentially distributed in nerve fibres innervating craniofacial tissues. Cephalalgia 28: 933–944, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram and Williams 1996.Ingram SL, Williams JT. Modulation of the hyperpolarization-activated current (Ih) by cyclic nucleotides in guinea-pig primary afferent neurons. J Physiol 492: 97–106, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juhasz et al. 2005.Juhasz G, Zsombok T, Jakab B, Nemeth J, Szolcsanyi J, Bagdy G. Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 25: 179–183, 2005. [DOI] [PubMed] [Google Scholar]
- Keller et al. 1989.Keller JT, Marfurt CF, Dimlich RV, Tierney BE. Sympathetic innervation of the supratentorial dura mater of the rat. J Comp Neurol 290: 310–321, 1989. [DOI] [PubMed] [Google Scholar]
- Kopp 2001.Kopp S Neuroendocrine, immune, and local responses related to temporomandibular disorders. J Orofac Pain 15: 9–28, 2001. [PubMed] [Google Scholar]
- Levy et al. 2007.Levy D, Burstein R, Kainz V, Jakubowski M, Strassman AM. Mast cell degranulation activates a pain pathway underlying migraine headache. Pain 130: 166–176, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maingret et al. 2008.Maingret F, Coste B, Padilla F, Clerc N, Crest M, Korogod SM, Delmas P. Inflammatory mediators increase Nav1.9 current and excitability in nociceptors through a coincident detection mechanism. J Gen Physiol 131: 211–225, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey and Gold 1999.McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol 61: 835–856, 1999. [DOI] [PubMed] [Google Scholar]
- Moore et al. 2002.Moore BA, Stewart TM, Hill C, Vanner SJ. TNBS ileitis evokes hyperexcitability and changes in ionic membrane properties of nociceptive DRG neurons. Am J Physiol Gastrointest Liver Physiol 282: G1045–G1051, 2002. [DOI] [PubMed] [Google Scholar]
- Oyelese and Kocsis 1996.Oyelese AA, Kocsis JD. GABAA-receptor-mediated conductance and action potential waveform in cutaneous and muscle afferent neurons of the adult rat: differential expression and response to nerve injury. J Neurophysiol 76: 2383–2392, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray and Wolff 1940.Ray B, Wolff H. Experimental studies on headache. Pain-sensitive structures of the head and their significance in headache. Arch Surg 41: 813–856, 1940. [Google Scholar]
- Renganathan et al. 2001.Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J Neurophysiol 86: 629–640, 2001. [DOI] [PubMed] [Google Scholar]
- Rozniecki et al. 1999.Rozniecki JJ, Dimitriadou V, Lambracht-Hall M, Pang X, Theoharides TC. Morphological and functional demonstration of rat dura mater mast cell–neuron interactions in vitro and in vivo. Brain Res 849: 1–15, 1999. [DOI] [PubMed] [Google Scholar]
- Salama et al. 1994.Salama H, Rigotti F, Gianserra R, Seibert J. The utilization of rubber dam as a barrier membrane for the simultaneous treatment of multiple periodontal defects by the biologic principle of guided tissue regeneration: case reports. Int J Periodont Restor Dent 14: 16–33, 1994. [PubMed] [Google Scholar]
- Sarchielli et al. 2000.Sarchielli P, Alberti A, Codini M, Floridi A, Gallai V. Nitric oxide metabolites, prostaglandins and trigeminal vasoactive peptides in internal jugular vein blood during spontaneous migraine attacks. Cephalalgia 20: 907–918, 2000. [DOI] [PubMed] [Google Scholar]
- Scholz et al. 1998.Scholz A, Gruss M, Vogel W. Properties and functions of calcium-activated K+ channels in small neurones of rat dorsal root ganglion studied in a thin slice preparation. J Physiol 513: 55–69, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu et al. 2007.Shimizu T, Toriumi H, Sato H, Shibata M, Nagata E, Gotoh K, Suzuki N. Distribution and origin of TRPV1 receptor-containing nerve fibers in the dura mater of rat. Brain Res 1173: 84–91, 2007. [DOI] [PubMed] [Google Scholar]
- Shin et al. 2002.Shin J, Cho H, Hwang SW, Jung J, Shin CY, Lee SY, Kim SH, Lee MG, Choi YH, Kim J, Haber NA, Reichling DB, Khasar S, Levine JD, Oh U. Bradykinin-12-lipoxygenase-VR1 signaling pathway for inflammatory hyperalgesia. Proc Natl Acad Sci USA 99: 10150–10155, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staikopoulos et al. 2007.Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. Localization of P2X2 and P2X3 receptors in rat trigeminal ganglion neurons. Neuroscience 144: 208–216, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart et al. 2003.Stewart T, Beyak MJ, Vanner S. Ileitis modulates potassium and sodium currents in guinea pig dorsal root ganglia sensory neurons. J Physiol 552: 797–807, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strassman et al. 1996.Strassman AM, Raymond SA, Burstein R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 384: 560–564, 1996. [DOI] [PubMed] [Google Scholar]
- Sugiura et al. 2002.Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol 88: 544–548, 2002. [DOI] [PubMed] [Google Scholar]
- Tu et al. 2004.Tu H, Deng L, Sun Q, Yao L, Han JS, Wan Y. Hyperpolarization-activated, cyclic nucleotide-gated cation channels: roles in the differential electrophysiological properties of rat primary afferent neurons. J Neurosci Res 76: 713–722, 2004. [DOI] [PubMed] [Google Scholar]
- Welch 2003.Welch KM Contemporary concepts of migraine pathogenesis. Neurology 61: S2–S8, 2003. [DOI] [PubMed] [Google Scholar]
- Werz and MacDonald 1983.Werz MA, MacDonald RL. Opioid peptides with differential affinity for mu and delta receptors decrease sensory neuron calcium-dependent APs. J Pharmacol Exp Ther 227: 394–402, 1983. [PubMed] [Google Scholar]
- Yamane et al. 2007.Yamane H, de Groat WC, Sculptoreanu A. Effects of ralfinamide, a Na+ channel blocker, on firing properties of nociceptive dorsal root ganglion neurons of adult rats. Exp Neurol 208: 63–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura and de Groat 1999.Yoshimura N, de Groat WC. Increased excitability of afferent neurons innervating rat urinary bladder after chronic bladder inflammation. J Neurosci 19: 4644–4653, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.