Abstract
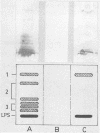
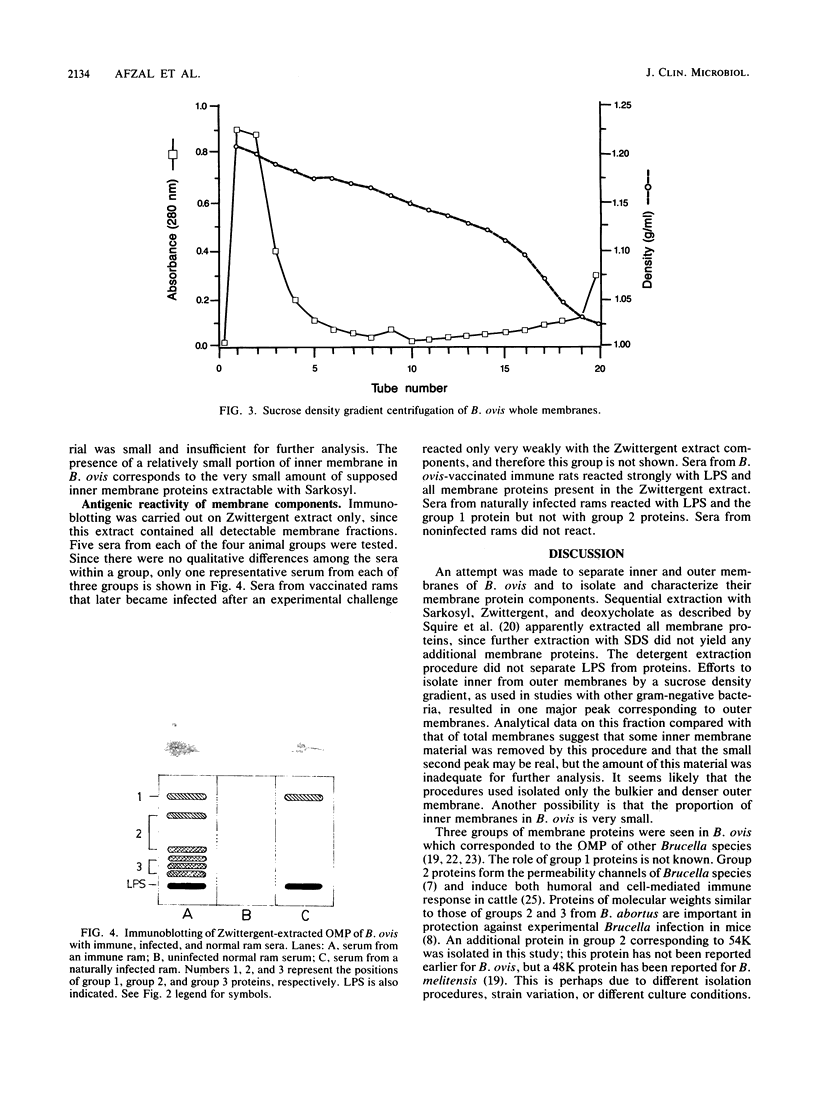
Brucella ovis cell membranes were isolated from fractured and lysozyme-treated cells by ultracentrifugation. These preparations appeared to consist largely of outer membranes, as judged from the results of ultracentrifugation experiments in sucrose density gradients under conditions that are widely used to separate inner and outer membranes of gram-negative bacteria. The sequential detergent extraction of cell membranes yielded mainly lipopolysaccharide and three groups of outer membrane proteins. In immunoblotting, lipopolysaccharide had good antigenic reactivity with all sera from rams exposed to B. ovis (vaccination or natural infection), but some outer membrane proteins reacted strongly only with sera from immune (vaccinated) rams, not from infected rams, suggesting a possible diagnostic role for such proteins in predicting immunity or infection.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamus G., Mulczyk M., Witkowska D., Romanowska E. Protection against keratoconjunctivitis shigellosa induced by immunization with outer membrane proteins of Shigella spp. Infect Immun. 1980 Nov;30(2):321–324. doi: 10.1128/iai.30.2.321-324.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzal M., Tengerdy R. P., Ellis R. P., Kimberling C. V., Morris C. J. Protection of rams against epididymitis by a Brucella ovis-vitamin E adjuvant vaccine. Vet Immunol Immunopathol. 1984 Oct;7(3-4):293–304. doi: 10.1016/0165-2427(84)90087-4. [DOI] [PubMed] [Google Scholar]
- Afzal M., Tengerdy R. P., Squire P. G., Ellis R. P. Characterization of Brucella ovis lipopolysaccharide and its use for diagnosis of ram epididymitis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984 Dec;20(6):1159–1164. doi: 10.1128/jcm.20.6.1159-1164.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D. T., Wilson B. L., Moreno E., Angus R. D., Jones L. M. Characterization of Brucella abortus soluble antigen employed in immunoassay. J Clin Microbiol. 1980 Apr;11(4):355–362. doi: 10.1128/jcm.11.4.355-362.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin J. C., Plant J. W., Claxton P. D. Evaluation of surface components of Brucella ovis as antigens for the detection of precipitin antibody in serums from artificially exposed rams. Aust Vet J. 1983 Sep;60(9):264–267. doi: 10.1111/j.1751-0813.1983.tb07100.x. [DOI] [PubMed] [Google Scholar]
- Douglas J. T., Rosenberg E. Y., Nikaido H., Verstreate D. R., Winter A. J. Porins of Brucella species. Infect Immun. 1984 Apr;44(1):16–21. doi: 10.1128/iai.44.1.16-21.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubray G., Bézard G. Isolation of three Brucella abortus cell-wall antigens protective in murine experimental brucellosis. Ann Rech Vet. 1980;11(4):367–373. [PubMed] [Google Scholar]
- Ellar D. J., Muñoz E., Salton M. R. The effect of low concentrations of glutaraldehyde on Micrococcus lysodeikticus membranes: changes in the release of membrane-associated enzymes and membrane structure. Biochim Biophys Acta. 1971 Jan 5;225(1):140–150. doi: 10.1016/0005-2736(71)90292-6. [DOI] [PubMed] [Google Scholar]
- Fernandes P. B., Kim C., Cundy K. R., Haung N. N. Antibodies to cell envelope proteins of Pseudomonas aeruginosa in cystic fibrosis patients. Infect Immun. 1981 Aug;33(2):527–532. doi: 10.1128/iai.33.2.527-532.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxen H., Valtonen M., Mäkelä P. H. Immunization with major outer membrane proteins in experimental salmonellosis of mice. Infect Immun. 1979 Sep;25(3):857–862. doi: 10.1128/iai.25.3.857-862.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loeb M. R., Smith D. H. Outer membrane protein composition in disease isolates of Haemophilus influenzae: pathogenic and epidemiological implications. Infect Immun. 1980 Dec;30(3):709–717. doi: 10.1128/iai.30.3.709-717.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mills S. D., Bradbury W. C. Human antibody response to outer membrane proteins of Campylobacter jejuni during infection. Infect Immun. 1984 Feb;43(2):739–743. doi: 10.1128/iai.43.2.739-743.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E., Berman D. T., Boettcher L. A. Biological activities of Brucella abortus lipopolysaccharides. Infect Immun. 1981 Jan;31(1):362–370. doi: 10.1128/iai.31.1.362-370.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyon I., Berman D. T. Isolation, purification, and partial characterization of Brucella abortus matrix protein. Infect Immun. 1983 Jan;39(1):394–402. doi: 10.1128/iai.39.1.394-402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riezu-Boj J. I., Moriyón I., Blasco J. M., Marín C. M., Diaz R. Comparison of lipopolysaccharide and outer membrane protein-lipopolysaccharide extracts in an enzyme-linked immunosorbent assay for the diagnosis of Brucella ovis infection. J Clin Microbiol. 1986 May;23(5):938–942. doi: 10.1128/jcm.23.5.938-942.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. M., Verstreate D. R., Perera V. Y., Winter A. J. Outer membrane proteins from rough strains of four Brucella species. Infect Immun. 1984 Oct;46(1):188–194. doi: 10.1128/iai.46.1.188-194.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire P. G., Smiley D. W., Croskell R. B. Identification and extraction of Pasteurella haemolytica membrane proteins. Infect Immun. 1984 Sep;45(3):667–673. doi: 10.1128/iai.45.3.667-673.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Winter A. J. Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles and antigenic relatedness among outer membrane proteins of 49 Brucella abortus strains. Infect Immun. 1984 Oct;46(1):182–187. doi: 10.1128/iai.46.1.182-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARAVDEKAR V. S., SASLAW L. D. A sensitive colorimetric method for the estimation of 2-deoxy sugars with the use of the malonaldehyde-thiobarbituric acid reaction. J Biol Chem. 1959 Aug;234(8):1945–1950. [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]