Fig. 3.
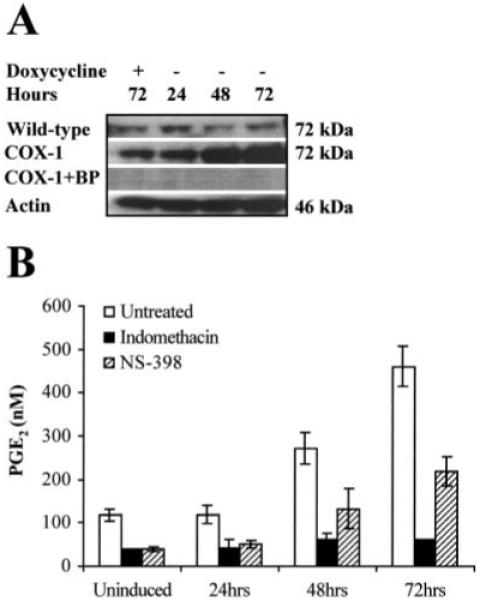
A, Western blot analysis of 20 μg of total protein isolated from wild-type HeLa Tet-Off and HeLa COX-1 Tet-Off cells grown for 24, 48, and 72 h, respectively, in the absence of DOX. In parallel, control uninduced HeLa COX-1 Tet-Off and wild-type HeLa Tet-Off cells were maintained for 72 h under the same conditions supplemented daily with DOX to a final concentration of 1 μg/ml. The proteins were loaded onto a 4-20% SDS-polyacrylamide gel, electrophoresed, and subsequently transferred to a PVDF membrane. The immunoblot was probed with antibody raised against the COOH terminus of human COX-1. A specific band of approximately 72 kDa was detected. No immunoreactivity was detected by preadsorbing the antibody with the blocking peptide (BP). COX-1 was normalized for protein loading against β-actin on the same blot. B, The functionality of the transfected COX-1 cDNA was assessed by ELISA, by measuring PGE2 secretion into the culture medium after COX-1 induction in the presence or absence of the COX enzyme inhibitor indomethacin, and treatment of HeLa cells with 5 μg/ml arachidonic acid.
