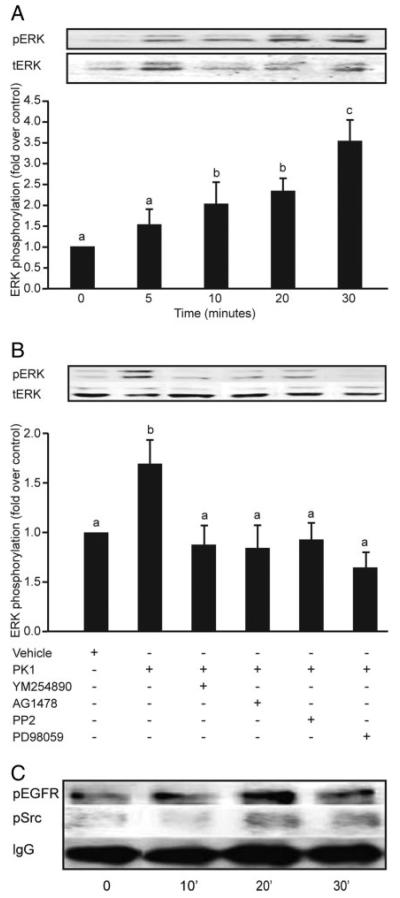
Fig. 5.
Phosphorylation of ERK, EGF, and cSrc in response to PK1 in third-trimester human placenta. Placental explants were incubated with 40 nm PK1 for 5, 10, 20, and 30 min and subsequently analyzed for phosphorylation of ERK1/2, cSrc, and EGFR (n = 3). A, There was a time-dependent increase in phosphorylation of ERK1/2 that peaked at 30 min (c is significantly different from a but not b; P < 0.05). Explants treated for 30 min with vehicle or 40 nm PK1 in the presence of chemical inhibitors of Gq (YM254890), EGFR kinase (AG1478), cSrc (PP2), or MEK (PD98059) were then subsequently analyzed for ERK phosphorylation (n = 7). B, There was a significant decrease in ERK 1/2 phosphorylation in explants incubated with inhibitors compared with explants treated with PK1 alone (b is significantly different from a; P < 0.05). Placental explants were stimulated with 40 nm PK1 for 0, 10, 20, and 30 min, and lysates were immunoprecipitated with anti-phosphotyrosine antibody and then subjected to Western immunoblot analysis using antibodies against cSrc and EGFR (n = 4). Treatment with 40 nm PK1 resulted in a 2-fold increase in phosphorylation of Src and EGFR within 20 min of treatment. C, IgG was used to demonstrate equal sample loading (representative blot). Data are shown as mean ± SEM.