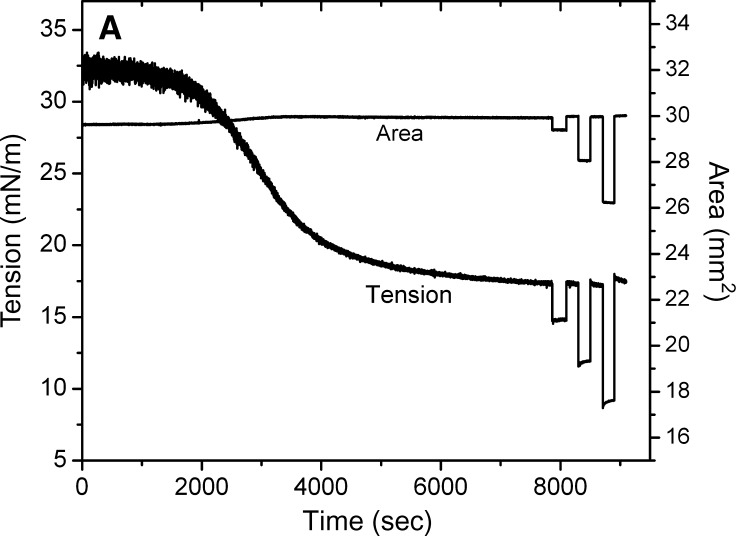
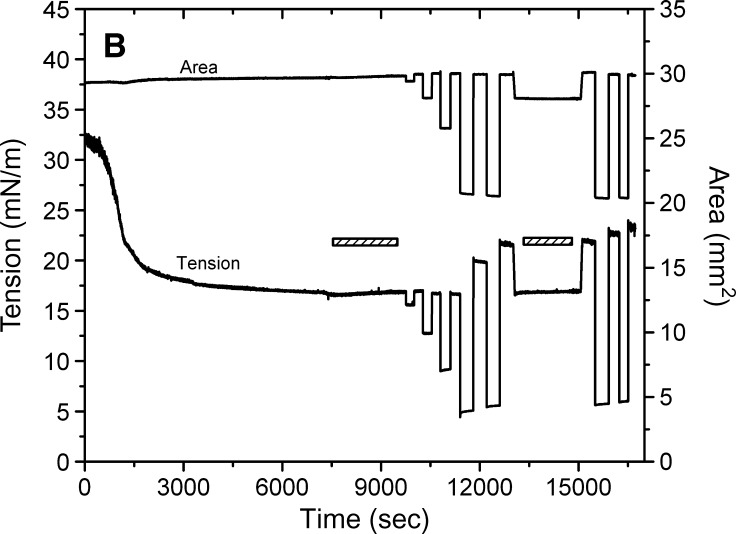
Fig. 4.
Examples of the interfacial tension (γ) and the area versus time curves for apoB[37–41] peptide at TO/W interface and the instant compression and re-expansion measurement before and after buffer exchange procedure. A: The instant compression and re-expansion was done after the tension approaching an equilibrium level without buffer exchange. A 16 μl triolein drop was formed in 2 mM pH 4.85 phosphate buffer. The concentration of apoB[37–41] peptide was 8.6 × 10−8 M in the aqueous phase. When γ reached the equilibrium level in about 7200 s, the 16 μl triolein drop was compressed by decreasing the volume by 1, 2, and 4 μl (corresponding to 2–16% changes in area), and then after several minutes re-expanded to the original volume 16 μl, respectively. B: The instant compression and re-expansion was done after the tension approaching an equilibrium level and after the buffer exchange. A 16 μl triolein drop was formed in 2 mM pH 4.85 phosphate buffer. The concentration of apoB[37–41] peptide was 1.3 × 10−7 M in the aqueous phase before buffer exchange. After approaching the equilibrium level, 150 ml buffer was exchanged (shown as the first bar in the figure). The peptide was virtually absent after the exchange. Then the 16 μl triolein drop was compressed by decreasing the volume by 1, 2, 4, and 8 μl (corresponding to 2–33% changes in area) and after several minutes re-expanded to the original volume 16 μl, respectively. Then the tension was lowered back to the equilibrium level by decreasing the volume at a rate of 0.02 μl/s, the second buffer exchange was carried out while the decreased volume was held (shown as the second bar in the figure). Then the volume was increased back to 16ul at a rate of 0.02 μl/s. Two 8 μl compressions and re-expansions were then made. All the measurements were carried out at 25 ± 0.1°C.