Abstract
In 2002, breast became the most common cancer site in Korean women. Using national breast cancer incidence data during 1993-2002, crude, age-standardized, and age-specific rates for incidence and mortality were calculated. Survival was examined for cases diagnosed during 1993-2002 and followed up to 2004. Observed survival was calculated using the life table method and relative survival using the Ederer II method. Age-standardized incidence rates in female increased from 14.5 in 1993 to 26.2 per 100,000 in 2002. Age-specific incidences showed peaks in women in their forties. Mortality rates increased from 3.7 in 1993 to 4.6 per 100,000 in 2002 and showed peaks in women in their fifties. Five-year relative survival for female breast cancer diagnosed during 1993-2002 was 82.2%. When we examined the secular trends using cases diagnosed 1993-1999 for complete 5-yr follow-up, the 5-yr relative survival increased from 75.2% in 1993 to 83.5% in 1999. The data from this study will provide valuable information to plan and evaluate actions against breast cancer including national breast cancer screening.
Keywords: Breast Cancer, Incidence, Mortality, Survival, Cancer Registry, Korea
INTRODUCTION
Breast cancer is the most common cancer in women worldwide regardless of the countries' level of development (1). Among Korean women, breast was the second most common cancer site following stomach in year 2001 (2). However, in 2002, it became the most common form of sporadic cancer in Korean women and comprised 16.8% of all female cancers (3). The Korean Ministry of Health and Welfare started a nationwide, hospital-based cancer registry called the Korea Central Cancer Registry (KCCR) in 1980. Details of the history, objectives, and activities of the KCCR were documented previously (3). In 1996, the Korea Breast Cancer Society (KBCS) initiated breast cancer on-line registration (4), which has been collecting data on breast cancer cases including stage information. The KCCR has been collaborating with the KBCS to improve completeness and validity of breast cancer registration. Breast cancer cases registered by the KCCR were merged with the cases registered by the KBCS. Also, through using data from 8 regional cancer registries and other sources including mortality data from the National Statistical Office (NSO), it became possible to produce national cancer statistics of Korea (2). In this paper, we present the trends of breast cancer incidence and mortality rates and the overall and stage-specific survival of breast cancer during 1993 and 2002 in Korea.
MATERIALS AND METHODS
Data sources
The breast cancer incidence database (DB) was derived from the Korea National Cancer Incidence Data Bases (KNCIDB), which were prepared by merging the KCCR mother DB, the population-based regional cancer registry (PB-RCR) DBs, the data from the ad hoc medical record review survey, cancer claims DB for newly diagnosed from the National Health Insurance Corporation (NHIC), the KBCS databases registered by surgeons and mortality data from the NSO for identifying the Death Certificate Only (DCO) cases and calculating mortality rates. In addition, information about carcinoma in situ (CIS) was ascertained from the KBCS registration.
Incidence
A total of 49,964 invasive breast cancer (ICD-9 code 174 for women and 175 for men; ICD-10 code C50) cases (49,558 women and 406 men) and 2,999 CIS cases (2,984 women and 15 men) were included for calculating incidence rates (Table 1). In this report, the denominator is the mid-year population annually released from the NSO (5). Crude incidence rates per 100,000 were calculated, and age-specific rates were calculated for 17 age groups (0-4, 5-9, every five year, 80 yr and over). All the rates were standardized to the WHO world standard population (6). Changes in the annual age-standardized breast cancer incidence rates were examined by calculating the average annual percentage change (APC) over a time period. For this, we used the Korea 2000 population as a standard population (5). The APC is 100 (eβ-1), where β is the slope from a regression of log age-standardized rates on calendar year (7). We calculated several indices of quality for incidence data registered; mortality:incidence ratio (M:I ratio), proportions of DCO, microscopically verified cases (MV%), and age-unknown cases (Age UNK%). For mortality:incidence ratio (M:I), an indicator of data completeness, the mortality data on cancer during the same period as the registered cases from the NSO were compared to the incidence data from the registry, which was presented in percent. DCO%, percentage of cases that are registered on the basis of death certificates only, is one of the indices of validity of diagnosis. The MV%, an indicator of the validity of the diagnostic information, is the percentage of cases for which the diagnosis was based on morphological verification of a tissue specimen.
Table 1.
Beast cancer incidence and mortality 1993-2002 in Korea
DCO, Death Certificate Only cases; CIS, Carcinoma in situ.
*Incidence rate: (A)+(B)/100,000 population; †Mortality rate: deaths/100,000 population.
Mortality
Crude and age-standardized mortality rates were calculated using the cases whose cause of death was breast cancer in NSO DB as a numerator and the mid-year population as a denominator. The average APC for mortality rates was also examined by the same method used for incidence rates.
Survival
Survival rates were examined for 46,985 female breast cancer cases first diagnosed during 1993-2002 and followed up for vital status through 31 December, 2004. Among 49,558 invasive cases, a total of 2,573 cases (5.2%) were excluded from the analysis; 1,786 DCO cases (3.6%) due to unverifiable date of diagnosis; 787 (1.6%) cases due to other errors related with stage and mismatched Personal Identification Number (PIN). The survival duration of each case was determined as the time difference (in months) between the date of initial diagnosis until the date of death, date of loss to follow-up, or the closing date for follow-up. To adjust for the effect of age to compare survival with those of other population, relative survival was truncated for the 0-74 yr age group and standardized to the standard cancer patient population (8). For calculating stage-specific survival rates, 24,447 cases with valid stage information were analyzed; 1,214 cases for stage 0, 6,445 cases for stage I, 13,116 cases for stage II, 2,978 cases for stage III, the 694 cases for stage IV following the American Joint Committee on Cancer 5th edition for staging (9). The registered cases whose cause of death was breast cancer were ascertained through the NSO, Ministry of Government Administration and Home Affairs, and the NHIC. Observed survival rates (OSR) were calculated using a Life table method, and relative survival rates (RSR) were examined by the Ederer II method (10) using an algorithm written in SAS by Paul Dickman (11) with some minor adaptations. The RSR confidence limits were derived by dividing the observed survival limits by the corresponding expected survival rate. To examine temporal trends, only cases diagnosed during 1993-1999 were analyzed, which were followed up for complete 5 yr.
RESULTS
Incidence & Mortality
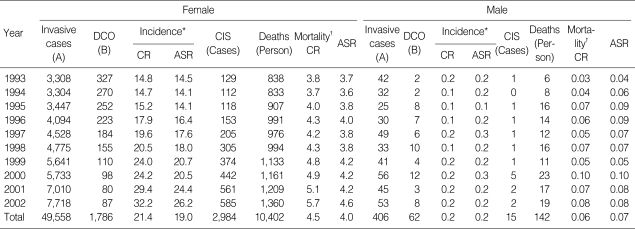
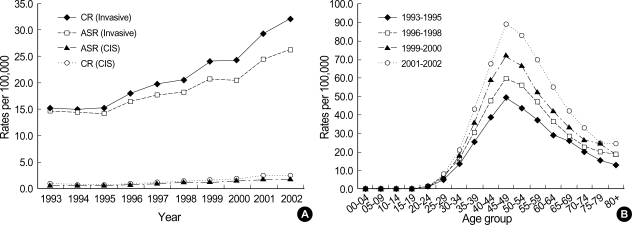
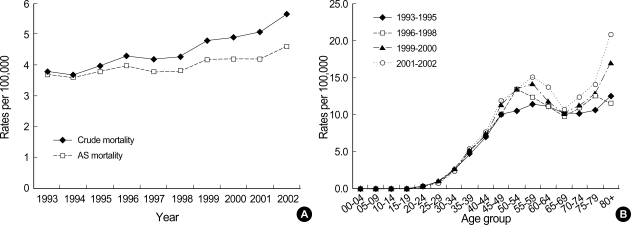
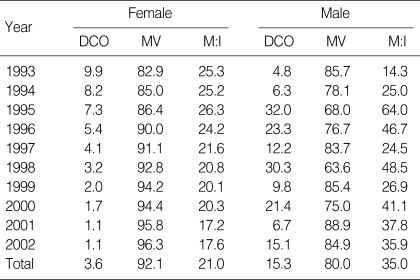
Among women, crude and age-standardized incidence and mortality rates using world standard population per 100,000 are presented in Table 1. Average APC of female invasive breast cancer incidence rates standardized to Korea 2000 standard population was 7.2% (p=0.002) from 1993 to 2002. CIS generally increased in women during the period (Fig. 1A). Age-specific incidence rates increased with age up to the 45-49 age group and leveled off at ages above that in women. Generally, peak incidence rates increased with time during 1993 and 2002 (Fig. 1B). Average APC of female invasive breast cancer mortality rates was 2.3% during the period, which was not significant (Fig. 2A). Age-specific mortality rates in women showed peaks in the 50-59 age group throughout the period and increased after the age of 60-69 (Fig. 2B). In men, the total number of incidence in Korea during the period of 1993-2002 was 406, which made a male-to-female ratio for breast cancer incidence 1:122 (406:49,558) during this period. Age-standardized incidence rates for men ranged from 0.2 to 0.3 per 100,000, and there was no visible trend during the period in either incidence or mortality possibly due to the limited number of cases. Although the overall incidence remain-ed low, age-specific incidence rates in men showed an increase with advancing age (Fig. 3). The quality indices of cancer incidence DB are summarized in Table 2. DCO%, especially of female invasive breast cancer kept decreasing from 9.9% in 1993 to 1.1% in 2002. MV% has been steadily increased from 84.0% in 1993 to 96.9% in 2002. The M:I ratio decreased during the period. There were no age-or sex-unknown cases.
Fig. 1.
Incidence trends of female breast cancer in 1993-2002. (A) Incidence trends of female invasive breast cancer and CIS by year of diagnosis. (B) Age-specific incidence rates of female invasive breast cancer by time period of diagnosis.
CIS, Carcinoma in situ; CR, Crude rate; ASR, Age-standardized rate using WHO world standard population.
Fig. 2.
Mortality trends of female breast cancer in 1993-2002. (A) Mortality trends of female breast cancer by year, (B) Age-specific mortality rates of female breast cancer by time period of death.
AS, Age-standardized using WHO world standard population.
Fig. 3.
Age-specific incidence rates for male invasive breast cancer by time period of diagnosis.
Table 2.
Quality indices of female invasive cancer DB by year of diagnosis (Unit: %)
MV, Microscopically verified; DCO, Death certificate only; M:I, Mortality: Incidence ratio.
Survival rates for invasive female breast cancer
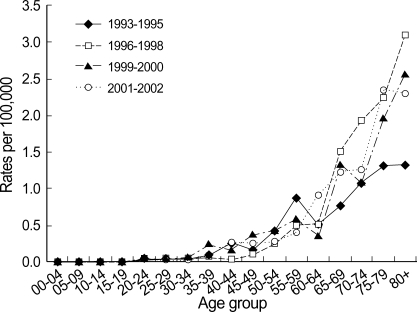
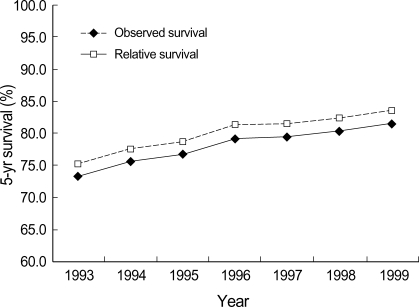
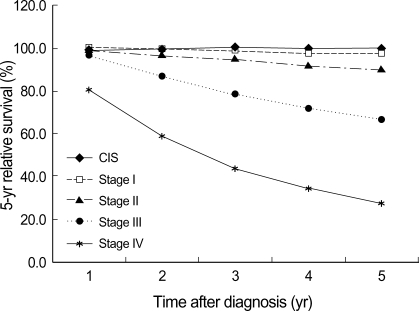
For survival analysis, only female cases were included. Median follow-up time was 55 months. Five-year OSR and RSR for invasive breast cancer diagnosed during 1993-2002 were 80.2% (95% CI 79.8, 80.6) and 82.2% (95% CI 81.8, 82.6), respectively, and age-standardized 5-yr RSR were 76.8% (95% CI 73.7, 81.2) for all ages and 81.0% (95% CI 79.9, 83.2) for the 0-74 age group. Five-year survival increased steadily from 1993 to 1999 (Fig. 4). Survival was highly dependent on the stage at diagnosis. Stage II was most common (53.6% on average) during the whole period. The proportion of early breast cancer (CIS and stage I) increased from 24.2% in 1993 to 36.6% in 2002 and showed the highest 5-yr RSR (Fig. 5).
Fig. 4.
Trends of observed and relative 5-yr survival rates for female invasive breast cancer diagnosed during 1993-1999.
Fig. 5.
Stage-specific 5-yr relative survival rates for female invasive breast cancer and CIS diagnosed during 1993-2002.
CIS, Carcinoma in situ.
DISCUSSION
This is the first report, that examined breast cancer trends over a decade based on population-based data in Korea. Although breast became the most common cancer site in Korean women in 2002, the level of breast cancer incidence in Korean women is considered as being modest when compared with other countries' incidences reported in the Globocan 2002. ASR per 100,000 Korean women in 2002 was lower than the world average (26.2 vs. 37.4) and lower than that of Japan (32.7), which is thought to have similar ethnic background (1). In terms of the age-specific incidence pattern, the rates showed a peak around the perimenopausal period, which was also found in Japan and Taiwan. This pattern is different from in Western countries, whose age-specific rates increased sharply up to the ages of menopause, but at a slower rate above that age (12, 13). The median age at diagnosis of invasive cancer in Korean women was 46 yr, which almost did not change during the 10-yr period. However, average APC for incidence rates standardized to Korea 2000 population was 7.2% in women. It seems to be much higher than those of European countries, which ranged from 0.8% to 3.0%, even after considering the use of different standard population (14). This increase might be caused partly by changes in previously known risk factors such as early menarche, late menopause, and late first-delivery (15, 16). However, it is more likely to reflect increasing completeness of registration.
The breast cancer mortality rate was the 5th highest in Korean women in 2004 (5). Korean women still have relatively low breast cancer mortality rates than any other countries (4.6 vs. 13.2 per 100,000), and there was no significant increase or decrease during the period (1). Age-specific mortality rates of female invasive breast cancer seem to be different from age-specific incidence rates. Considering there was no significant difference in the distribution of disease stage between age groups (data not shown), this might reflect that cause of death for a large proportion of patients could be assigned as other competing diseases. For age-specific mortality curves, only deaths from breast cancer were considered.
Survival rates of Korean female breast cancer were reported as 2nd highest (5-yr RSR 77.5%), only second to thyroid cancer (95.1%) (17). Compared with survival of other developing countries using age-standardized 5-yr RSR, our survival is slightly lower than that of Hong Kong, China (82.5%), but higher than those of Turkey (77.2%), Singapore (76.4%), Thailand (62.9%), and India (51.6%) (18). For European countries, from EUROCARE-3 study, age-standardized 5-yr RSR for Europe, based on 256,464 female breast cancer patients diagnosed during 1990-1994 from 22 European countries, was 76.1% (19), which is similar to that of Korea during 1993-1997. However, there is almost a 5-yr gap in terms of the diagnosis period between these, which needs to be taken into consideration. In our study, when the study period was split into two (1993-1997 vs. 1998-2002) for comparing the survival experience during the period, 5-yr RSR during the latter period (84.3%, 95% CI 83.8, 84.8) is higher than that (79.1%, 95% CI 78.5, 79.8) of the former period by 5.4%. It is very likely that the survival of these European countries improved during those 5 yr as our survival did during the study period. Also, using crude 5-yr RSR among American women diagnosed during 1996-2002, survival (89.0%) (7) is higher than that of Korea during 1998-2002. The five-year RSR of breast cancer among Japanese women diagnosed during 1993-1996 was 83% (20), which was higher than that of Korea during the similar period. However, we should interpret these results with great caution, since there are differences in the diagnosis period and standardization methods among populations. For stage-specific survival, 50.4% (24,867 out of 49,304 cases) of cases were excluded due to missing stage information. There is a difference in overall 5-yr RSR between cases with stage information and cases without by 9.8%; 87.2% (95% CI 86.6, 87.7) vs. 77.4% (95% CI 76.8, 78.0). We checked the systematic differences between staged and un-staged cases using other available information such as the mean age at diagnosis, treatment (operation/non-operation), and residential areas (data not shown). We found that the proportion of staged cases was increasing during the study period, which could represent cases with more favorable prognosis with time. Also surgical treatment was more common in staged patients. This phenomenon was observed previously and been interpreted that stages of who had not undergone surgery were more likely to be advanced than cases with stage information, which could cause the worse overall survival in un-staged cases (21).
Completeness is an important issue in calculating and interpreting cancer incidence, which we checked indirectly using DCO% and M:I ratio (22). However, DCO% itself is not an indicator of completeness, with elevated DCO% being suggestive of incompleteness. DCO%, especially of female invasive breast cancer, kept decreasing from 9.9% in 1993 to 1.1% in 2002 (2). It made very difficult to interpret the secular trends of incidence, and the estimated APC is very much likely to be overestimated. The M:I ratio has been decreased, which suggested improved completeness of incidence DB and correctly reflected the changing pattern of survival during the period at the same time. Especially, an ad hoc medical record review survey covering data registered from 1999 has been performed since 2001, which increases completeness. Another important aspect of registry data is validity, which we checked using MV%. MV% has been steadily increased from 84.0% in 1993 to 96.9% in 2002.
For survival analysis, we achieved nearly 100% follow-up through the PIN, used in Korea, which is given at the time of birth and contains information about sex, date, and place of birth. The accuracy of cause of death for breast cancer was reported to be very high (23), and the proportion of cause of death certified by doctors has increased up to 86.5% (24) in 2005. Additionally, cause of death has become more accurate and complete by collaboration between KCCR and NSO since 2000.
In summary, the female breast cancer incidence in Korea seems to have been increasing, which is possibly due to the combined effects of various factors including completeness of registry data, population aging, and changes in risk factors. Contributing factors should be identified by further studies, which will enable to interpret the registry data properly and to plan effective protective measures. It is also necessary to examine the factors related to improved survival including patient characteristics, disease stage, and treatments. This report will be valuable as baseline data to plan and evaluate actions against breast cancer including evaluating the national breast cancer screening program.
ACKNOWLEDGMENT
The authors would like to thank all the members who submitted data to KCCR and KBCS's online registry.
Footnotes
This work was supported by a grant 0410010-3 from the National Cancer Center, Korea.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, Noh HI, Pisani P, Park JG, Ahn YO, Lee SY, Lee CW, Woo ZH, Lee TY, Choi JS, Yoo CI, Bae JM. Nationwide cancer incidence in Korea, 1999-2001; first result using the National Cancer Incidence Database. Cancer Res Treat. 2005;37:325–331. doi: 10.4143/crt.2005.37.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin HR, Jung KW, Won YJ, Park JG 139 KCCR-affiliated Hospitals. 2002 Annual report of the Korea Central Cancer Registry: Based on registered data from 139 hospitals. Cancer Res Treat. 2004;36:103–114. doi: 10.4143/crt.2004.36.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korean Breast Cancer Society. Clinical characteristics of Korean breast cancer patients in 1998. J Korean Med Sci. 2000;15:569–579. doi: 10.3346/jkms.2000.15.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Statistical Office. 2006. [Accessed 7 September 2006]. Available at http://kosis.nso.go.kr.
- 6.Segi M. Cancer Mortality for selected sites in 24 countries (1950-1957) Sendai, Japan: Tohuku University of Medicine; 1960. [Google Scholar]
- 7.Ries L, Harkins D, Krapcho M, Mariotto A, Miller B, Feuer E, Clegg L, Eisner M, Horner M, Howlader N, Hayat M, Hankey B, Edwards B. SEER Cancer Statistics Review, 1975-2003. Bethesda MD: National Cancer Institute; [Accessed 12 September 2006]. Available at http://seer.cancer.gov/csr/1975_2003/ based on November 2005 SEER data submission, posted to the SEER web site, 2006. [Google Scholar]
- 8.Sankaranarayanan R, Black RJ, Swaminathan R, Parkin DM. An overview of cancer survival in developing countries. IARC Sci Publ. 1998;145:135–173. [PubMed] [Google Scholar]
- 9.American Joint Committee on Cancer. AJCC Cancer staging manual. 5th ed. Philadelphia, USA: Lippincott-Raven; 1997. [Google Scholar]
- 10.Ederer F, Heise H. Instructions to IBM programmers in processing survival computations. methodological note No.10. Bethesda MD: National Cancer Institute; 1959. [Google Scholar]
- 11.Dickman P. [Accessed 6 September 2006]. Available at http//www.pauldickman.com.
- 12.MacMahon B. Epidemiology and the causes of breast cancer. Int J Cancer. 2006;118:2373–2378. doi: 10.1002/ijc.21404. [DOI] [PubMed] [Google Scholar]
- 13.Parkin DM, Whelan SL, Ferlay J, Storm H. Cancer incidence in five continents. vol VIII. Lyon, France: IARC; 2002. [Google Scholar]
- 14.Botha JL, Bray F, Sankila R, Parkin DM. Breast cancer incidence and mortality trends in 16 European countries. Eur J Cancer. 2003;39:1718–1729. doi: 10.1016/s0959-8049(03)00118-7. [DOI] [PubMed] [Google Scholar]
- 15.Korean Breast Cancer Society. Nationwide Korean breast cancer data of 2002. J Korean Breast Cancer Soc. 2004;7:72–83. [Google Scholar]
- 16.Yoo KY, Kang D, Park SK, Kim SU, Kim SU, Shin A, Yoon H, Ahn SH, Noh DY, Choe KJ. Epidemiology of breast cancer in Korea: occurrence, high-risk groups, and prevention. J Korean Med Sci. 2002;17:1–6. doi: 10.3346/jkms.2002.17.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae JM, Won YJ, Jung KW, Suh KA, Yun YH, Shin MH, Ahn YO, Lee DH, Shin HR, Ahn DH, Oh DK, Park JG 134 KCCR-affiliated Hospitals. Survival of Korean Cancer Patients Diagnosed in 1995. Cancer Res Treat. 2002;34:319–325. doi: 10.4143/crt.2002.34.5.319. [DOI] [PubMed] [Google Scholar]
- 18.Sankaranarayanan R, Swaminathan R, Brenner H. Cancer survival in developing countries: an overview. Union Internationale Contre le Cancer (UICC) World Cancer Congress; 2006. [Google Scholar]
- 19.Coleman MP, Gatta G, Verdecchia A, Estéve J, Sant M, Storm H, Allemani C, Ciccolallo L, Santaquilani M, Berrino F EUROCARE Working Group. EUROCARE-3 summary: cancer survival in Europe at the end of the 20th century. Ann Oncol. 2003;14(Suppl 5):v128–v149. doi: 10.1093/annonc/mdg756. [DOI] [PubMed] [Google Scholar]
- 20.Nomura K, Sobue T, Nakatani H, Maehara M, Kiryu Y, Tsukuma H, Doi M. Cancer statistics in Japan 2005. Tokyo, Japan: Foundation for Promotion of Cancer Research; 2005. [Google Scholar]
- 21.Sant M, Allemani C, Berrino F, Coleman MP, Aareleid T, Chaplain G, Coebergh JW, Colonna M, Crosignani P, Danzon A, Federico M, Gafà L, Grosclaude P, Hédelin G, Macè-Lesech J, Garcia CM, Møller H, Paci E, Raverdy N, Tretarre B, Williams EM European Concerted Action on Survival and Care of Cancer Patients (EUROCARE) Working Group. Breast carcinoma survival in Europe and the United States. Cancer. 2004;100:715–722. doi: 10.1002/cncr.20038. [DOI] [PubMed] [Google Scholar]
- 22.Parkin DM, Chen VW, Ferlay J, Galceran J. Comparability and Quality Control in Cancer Registration. Lyon: IARC; 1994. [Google Scholar]
- 23.Lee DH, Shin HR, Ahn DH, Chun BY, Kam Sin, Ahn YO. Accuraey of Cancer Death Certificates in KOREA: A comparison between diagnoses in the central cancer registry and certified underlying causes of death. J Korean Cancer Assoc. 2000;32:210–219. [Google Scholar]
- 24.Ministry of Health and Welfare. 2005 Annual report on the cause of death statistics (based on vital registration) 2006. [Google Scholar]