Abstract
Male courtship in fruit flies is regulated by the same major regulatory genes that also determine general sexual differentiation of the animal. Elaborate genetics has given us insight into the roles of these master genes. These findings have suggested two separate and independent pathways for the regulation of sexual behavior and other aspects of sexual differentiation. Only recently have molecular studies started to look at the downstream effector genes and how they might control sex-specific behavior. These studies have confirmed the essential role of the previously identified male specific products of the fruitless gene in the neuronal circuits in which it is expressed. But there is increasing evidence that a number of non-neuronal tissues and pathways play a pivotal role in modulating this circuit and assuring efficient courtship.
Key Words: Courtship, fruitless, doublesex, fat body, takeout, sex determination, Drosophila.
INTRODUCTION
One of the fascinating fields of neurogenetics is the study of complex behaviors and the genes that control them. Courtship behavior in fruit flies (Drosophila melanogaster) is particularly well suited for such studies. The behavior consists of a series of consecutive steps that the courting male performs and that can easily be observed and quantified (reviewed in [1,2]): The male orients himself toward the female, taps the female with his forelegs, extends and vibrates one wing to “sing” a courtship song, licks the female’s genitalia, attempts copulation, and copulates. Thanks to the many genetic and molecular tools that exist for this model organism it has been possible to gain significant insight into the genes and processes that regulate the behavior.
In Drosophila melanogaster development, sex is determined cell- autonomously or by signals between adjacent tissues and not by hormones (reviewed by [3,4]). Sexual behavior in flies is regulated by the same master regulators that control general somatic sexual development and are part of a cascade of alternative splicing events. The primary signal lies in the ratio of X-chromosomes to autosomes, which determines whether a functional form of the “master regulator” protein Sex-lethal (Sxl) is produced (in females) or not (in males) (Fig. 1). In females, functional Sxl protein acts as a splicing regulator to control female-specific expression of the transformer (TraF) protein, itself a splicing regulator. TraF interacts with Tra-2, another splicing regulator. Together they control the female-specific splicing of doublesex (dsx) and fruitless (fru) pre-mRNAs. This results in the production of the female-specific dsx protein (DSX-F). No female specific FRU protein is formed because of translational control [5,6]. In males, the absence of TraF leads to the default splicing of both dsx and fru RNAs and to the production of male-specific dsx (DSX-M) and FRUM proteins. The central role of tra in the control of sexual differentiation and sex specific behavior is demonstrated by the fact that chromosomal females with a mutation in the tra gene are transformed into normal males with male courtship behavior [7].
Fig. (1).
Simplified version of the Drosophila sex determination pathway.
Since tra controls both dsx and fru, further studies have examined which one of them controls mating behavior by examining the courtship behavior of dsx and fru mutant males. A mutation in dsx was found to reduce overall male courtship and to impair courtship song, but it did not abolish courtship [8,9]. Females that expressed male DSX-M acquired male morphology, but did not court [8]. In contrast, males with strong mutant alleles of fru barely courted, demonstrating that fru is essential for male courtship. Weaker fru mutations lowered courtship and caused males to indiscriminately court females and males [10-14]. Based on these and similar experiments it was proposed that there are two independent branches downstream of tra, one through dsx that controls somatic sexual differentiation outside the nervous system, and another through fru in the nervous system that controls male courtship behavior [15]. In recent years it has become increasingly evident, however, that the two pathways are both significantly contributing and interacting to regulate male courtship through both the CNS and other tissues. This article will review the role of fru in regulating courtship and discuss recent evidence that there is a close interplay between dsx and fru regulated pathways and genes in the regulation of courtship.
FRU IS A MASTER REGULATOR OF MALE SPECIFIC BEHAVIOR
Recent excellent reviews have described the complexities of the fru gene and its functions in detail [16-18]. This article will summarize some of this information and focus on more recent findings on how FRUM may control an amazing array of behaviors. A central role for fru in male courtship has recently been confirmed by findings that FRUM and the neuronal network defined by FRU expressing neurons are sufficient to specify the early steps of male mating behavior [19,20]. Expression of FRUM in otherwise completely normal females leads to male courtship behavior towards other females, although at lower levels than in control males and with impaired courtship song, indicating that factors other than FRUM are also required. In addition, females that express FRUM show male specific aggression, another sex specific behavior that is regulated by fru [21-23].
The fruitless gene is large (150 kb) and encodes numerous transcripts with non-sex-specific and sex-specific functions that are transcribed from several promoters [6,11-13,24]. The most distal promoter, P1, gives rise to the sex specific transcripts. They contain TraF binding sequences in their second exon. Binding of TraF, which is only present in females, leads to the choice of an alternative 5’ splice site and inclusion of sequences with numerous translational stop codons. This female specific transcript appears to be unable to produce any protein [6]. In males, in the absence of TraF binding, the stop codon containing part of the transcript is spliced out, thus allowing a long uninterrupted reading frame that gives rise to the male specific FRUM protein [6,11-13,25]. Fru proteins belong to the BTB-Zn-finger protein family, suggesting that they act as transcription factors, although no direct molecular targets have been identified yet. However, genome-wide searches for genes that are controlled by fru have identified numerous target genes (see later). The male specific FRUM protein contains a unique 101 amino acid N-terminal region. These sequences are highly conserved among Drosophila species. Their male specific function is still under investigation. A recent report has suggested that these residues are essential to allow FRUM to function when it is ectopically expressed in otherwise normal females, but that they may be less important for FRUM function in its normal male context [26]. It has already been demonstrated that FRUM isoforms that contain one of several alternative putative DNA binding domains affect male neuronal differentiation and behavior differently [27].
The FRUM protein is expressed in about 2000 neurons of the brain and ventral ganglia, as well as in the peripheral nervous system [5,11-13,19,20,28,29]. Are these neurons unique to males, and is this how FRU exerts its functions? That this is not the case was recently shown by the generation of transgenic flies that contained a manipulated fru gene that exclusively spliced the sex specific transcript in a male mode, even in females. To visualize the protein made from this transcript, FRU coding sequences were replaced with sequences coding for the yeast transcription factor Gal4 whose expression can be visualized, thus marking cells that usually express the male specific splice form. When this transgene was expressed in females, the expression pattern was basically indistinguishable from the pattern normally seen in males, indicating that the neuronal circuits that express FRUM in males are present in females [19,20,28]. Therefore, there are no gross anatomical differences caused by FRUM expression that can account for fru dependent male behaviors. However, on a smaller scale, neuronal dimorphism may be part of fru regulation. There are several FRUM expressing clusters that differ in males and females by cell number and other characteristics. And there is increasing evidence for specific roles for these and other subsets of FRUM expressing cells. A cluster of FRUM expressing neurons that are part of the median bundle, a structure that receives sensory input, is involved in controlling the sequential order of the different courtship steps, perhaps by coordinating different sensory stimuli [30]. Two glomeruli in the antennal lobe which receive olfactory input (DA1 and VA1v) differ in size between males and females, and those two glomeruli, plus an additional one (VL2A), were found to be the only olfactory glomeruli that were innervated by fru-Gal4 positive neurons [20,31]. Olfactory neurons that project to the DA1 glomerulus express the Or67d olfactory receptor which responds to 11-cis-vaccenyl acetate, a male derived pheromone (cVA). Activation of the receptor with cVA has different functions in males and in females: In males, it inhibits courtship to other males, in females it acts to stimulate receptivity towards males [32,33]. Recent experiments have shown how the same pheromone perceived by the same receptor might lead to different behaviors in males and females. The projections from the DA1 glomerulus to the protocerebrum, a higher order brain center, were found to be sexually dimorphic. The male specific projection pattern is dependent on the expression of FRUM in these neurons and other FRUM positive cells [34]. In yet another cluster in the brain, named fru-mAL, neuron number and morphology is different between males and females. These differences depend on FRUM and its regulation of differential programmed cell death between males and females [29]. Intriguingly, this cluster of neurons has recently been implicated in the control of male specific aggressive behavior [35].
These data demonstrate that fru expressing clusters can have distinct male specific functions. It is not known how this functional specificity is brought about. Part of the specificity might be due to the fact that these clusters are part of different and dedicated neuronal circuits. Since FRUM has the characteristics of a transcription factor it is likely to bestow male specific molecular characteristics to the neurons that express it. This could occur both during the development of these neurons and/or by setting differential physiological states of individual neurons in the adult animal. Whether the same set of FRUM-dependent transcripts is induced in all fru expressing neurons or whether subsets of fru clusters express specific signatures of fru regulated genes remains to be seen.
BOTH FRUM AND DSX ARE REQUIRED IN THE CNS FOR MALE SPECIFIC FUNCTIONS
The male courtship song is an important part of male courtship behavior that has been shown to map to certain regions of the brain and the ventral thoracic ganglia [36]. fru mutant males have impaired courtship song, indicating a role for fru in regulating the behavior [13,14]. FRUM however does not appear to be sufficient for specifying normal courtship song, since females expressing FRUM do not exhibit normal courtship song [37]. Since a mutation in dsx also causes impaired courtship song in males, Rideout et al. and others tested the possibility that both fru and dsx are required to specify normal male courtship song [8,37]. Indeed, expression of both FRUM and the male form of DSX, DSX-M, was required for normal courtship song. Co-expression of FRUM and DSX-M was observed in neurons of the mesothoracic ganglia in a neuronal cluster that shows a sexually dimorphic number of FRUM expressing neurons [37]. Intriguingly, expression of DSX-M was required to obtain the full set of male FRUM expressing neurons. This is reminiscent of previous findings that both DSX-M and FRUM are required in the abdominal ganglia for the differentiation of male-specific serotonergic neurons [27], and that DSX-M is required for an increased number of neurons in the abdominal ganglia of males [38]. A central role for FRUM expressing abdominal neurons in the production/performance of courtship song was shown recently by Clyne et al. [39]. The authors used a light-activated ion-channel that they expressed in all fru-expressing neurons. This allowed them to specifically activate these neurons by light. When the cells in the abdomen of decapitated flies were activated, both males and females extended a wing and performed courtship song, although the characteristics of the song were different in females. When the females also expressed FRUM, the displayed song was very male-like and was recognized by control females as valid courtship song. The authors concluded that the potential to display the behavior was largely present in both sexes, but whether it was initiated, and the quality of the song was dependent on stimuli and/or coordination mediated by FRUM. In contrast to the results obtained in decapitated flies, light activation of the behavior occurred at very low frequency in intact flies. Since control of courtship song does not only require male abdominal ganglia, but also male posterior regions of the brain, it is possible that the light-activated response was suppressed in intact flies, because sensory stimuli that usually trigger the behavior were absent and higher-order control neurons were therefore inhibiting the display of the behavior.
That females may possess some intrinsic neural pathways for courtship has previously been suggested by findings that females which lack FRUM, but are mutant for the gene retained (retn), show some male courtship [40]. retn codes for a ARID-box transcription factor that is expressed in a small subset of neurons in both males and females that does not overlap with fru expressing neurons. Furthermore, the effect of retn is influenced by whether DSX-M or DSX-F is present in these flies and the authors showed that fru and dsx can act together in the context of developmental genes such as retn.
DSX-M was also found to control the expression of a male-specific gustatory receptor, Gr68a. It is expressed in taste sensillae on the male foreleg and may play a role in the pheromonal perception of females. Removal of Gr68a by RNAi affects courtship [41].
THE FAT BODY, A NON-NEURONAL TISSUE, AND GENES EXPRESSED OUTSIDE THE FRU CIRCUITS ARE REQUIRED FOR NORMAL COURTSHIP
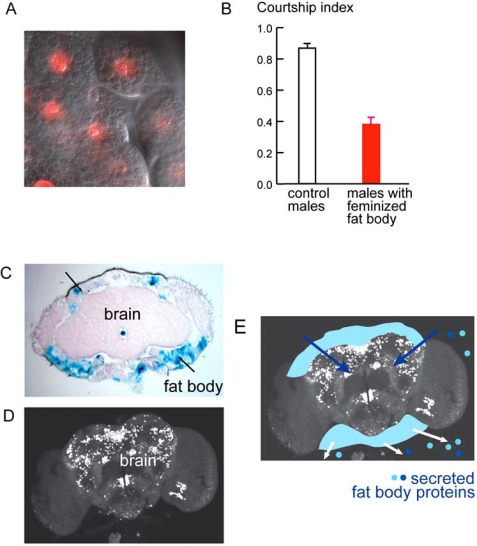
Both FRUM and DSX are transcription factors, but very little is known about the sex-specific genes they regulate and what role they might play in courtship. Their identification is crucial for our understanding of courtship regulation. Several groups have performed molecular screens to identify sex specific transcripts and transcripts that change in fru and dsx mutants [41-45]. However, the biological role of only a few of these transcripts has been examined so far. The takeout (to) gene was identified in a subtractive screen and was shown to be preferentially expressed in male heads [44]. A mutation in takeout affects male courtship behavior and interacts genetically with fru, indicating that they act in the same overall pathway that regulates mating behavior. takeout mutant males showed an overall reduction in courtship; although they were able to perform all steps of courtship, they initiated and maintained the behavior at a significantly lower rate. Given that the mutant affects mating behavior, it was surprising when it was found that the takeout transcripts were not present in the nervous system, but that the gene was male-specifically expressed in the fat body that surrounds the brain (there is also some non-sex specific expression in the antennae, the olfactory organs of the fly) [44]. The insect fat body consist of large, lipid-filled cells and is often compared to the mammalian liver (Fig. 3). Its crucial role in fat storage, energy metabolism and immunity is well documented [46-48], but it had not been implicated in the control of sex specific behaviors before. Its only known sex specific role was in the production of yolk proteins in females [49]. To test whether there is a general sex specific role for the fat body in male courtship behavior, genetic means were used to feminize the fat body in otherwise normal males and to ask whether this affected courtship. To do so, the female specific TraF protein was targeted only to fat body cells. To change sex only in a defined subset of cells is feasible in flies because, as mentioned earlier, sex is determined cell-autonomously and not regulated by circulating hormones. Courtship was reduced drastically in males with feminized fat body, indicating that the sexual identity of the fat body is indeed crucial for normal courtship [50]. Interestingly, courtship in these males was considerably lower than in the takeout mutants, suggesting that the feminization did not just reduce the amount of takeout, but probably also that of other fat body transcripts which normally play a role in courtship regulation. These other transcripts remain to be identified. The lower courtship scores observed in males with feminized fat body are reminiscent of the reduced scores observed in females that express FRUM. What if courtship in FRUM females was lower than normal because they still had a female fat body? In a genetic experiment that did the opposite of the one just described in males, the fat body tissue was masculinized in females that also express FRUM. These females now courted as well as normal males, underscoring the importance of the fat body and its interaction with the CNS [50]. There is increasing evidence for a sex specific role of fat body factors. In another screen for sex specifically expressed transcripts in the head, Fuji et al. [41] identified four genes with preferential sex specific expression in the fat body. tsx, sxe1, sxe2 were male-specifically, and fit was female specifically expressed. In addition, recent genomic screens have identified a number of sex-specifically expressed genes that appear to be expressed in the fat body [45], see below.
Fig. (3). Sex specific factors from the fat body are essential for courtship.
(A) Fat body cells are large and contain lipid droplets (for visualization, the nuclei were marked with a fluorescent protein (dsRed Stinger). (B) Courtship performance of wildtype males and males with feminized fat body. The courtship index is the fraction of time a male courts within the 10 minute observation period. n=10, error bars indicate s.e.m. (C) Frontal section of a fly head. Fat body cells are marked by X-Gal staining (blue, arrows). The fat body surrounds the brain. (D) Isolated whole brain showing fru expressing cells (picture courtesy of Dornan et al. 2005). (E) Model for fat body – brain interaction. It is proposed that the fat body secretes factors that enter the hemolymph, the circulating fluid, reach the brain and interact with fru-expressing neuronal circuits (Modified with permission from Dauwalder et al. Genes & Dev., Nov 2002; 16: 2879-2892, Copyright 2002, Cold Spring Harbor Laboratory Press, Lazareva et al. PLoS Genet 3(1): e16. doi:10.1371/journal.pgen.0030016) and Dornan et al. Genesis 2005; 42:236–246, copyright 2005, Wiley-Liss, Inc. Reprinted with permission of Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc).
How can a tissue like the fat body regulate courtship behavior? As discussed earlier, expression of FRUM in the CNS is required to establish the competence for courtship behavior. Obviously, fat body factors need to interact with the nervous system to regulate its function. Since the fat body is a major secretory tissue, one possibility is that it does so by secreting factors into the hemolymph, the circulating fluid of flies, and that these factors somehow interact with the brain. Consistent with this hypothesis is the finding that the Takeout protein is present in the hemolymph [50]. This suggests that soluble, circulating factors may play a significant role in the control of Drosophila sexual behavior, reminiscent of the hormonal control of behavior in vertebrates (Fig. 3). How such proteins cross through or signal through the blood brain barrier and interact with fru circuits is unknown. Two lines of evidence suggest that the sex-specific role of fat body factors is physiological, in the adult and behaving fly, rather than during development: Only feminization of fat body in adult flies, but not at larval stages, leads to the described reduction in courtship [50]. And, in experiments that looked at transcriptional changes in adult males that were allowed to court females for 5 minutes, at least three out of eleven up-regulated genes were genes that are controlled by the sex determination pathway and are expressed in the fat body [51].
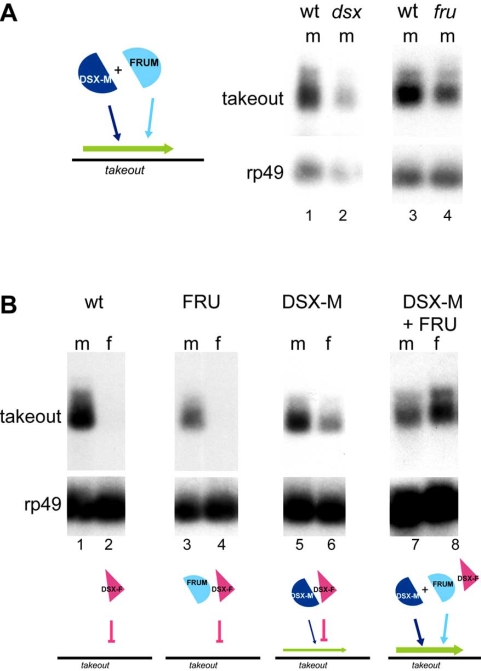
The takeout gene codes for a 27kD protein with characteristics of soluble carrier proteins that is most similar in sequence to secreted Juvenile Hormone binding proteins of other insects [44,52,53]. Interestingly, expression of the takeout gene is regulated by both DSX-M and FRU. Mutations in either gene reduce the amount of takeout present in males, indicating that both fru and dsx are required for full takeout activation [44] (Fig. 2). These findings support the notion that DSX-M acts as an activator in males, as had been previously suggested [54-56]. The action of DSX proteins is best characterized in the case of the female specific yolk protein (yp2) promoter. Both DSX-F and DSX-M bind the yp2 promoter; bound DSX-F activates transcription, whereas bound DSX-M represses its activation [56-58]. Thus, the described effects of DSX proteins on yp2 are opposite to those observed for takeout regulation. In contrast to the regulation observed in yp2, however, both DSX-M and FRUM are required for normal takeout expression. Consistent with this, expression of FRUM in females alone is not sufficient for male levels of takeout, most likely due to the absence of DSX-M and an inhibitory effect by the presence of DSX-F (Fig. 2). Only in females that express both FRUM and DSX-M are wildtype levels of takeout expression observed. It is not known yet whether DSX and FRU act by directly binding to the takeout promoter, or through other transcription factors. Potential DSX consensus binding sites [59] have been observed within 1kb upstream of the takeout transcription start site. FRU recognition and binding sequences have not been described yet.
Fig. (2). takeout expression requires both doublesex and fruitless.
(A, B) Northern analysis of takeout expression and cartoon interpretation of takeout transcriptional regulation in males (A) and females (B). Note that while both DSX-M and FRUM are required there is no evidence that they bind directly or interact physically with each other. (A) takeout expression in dsx1 (lane 2) and fru4/fru3 mutant males is reduced (lane 4), when compared to their heterozygous siblings (lanes 1 and 3). (B) Forced expression of male-specific forms of dsx and fru act synergistically to induce takeout in XX individuals. Females expressing the male DSX-M from the dominant mutation dsxSWE show activation of takeout (lane 6) compared to control females (lane 2). These females are dsxSWE/ dsx+ and produce both dsxF and dsxM. There is no effect of dsxSWE/ dsx+ on takeout expression in males (compare lanes 5 and 1). No significant induction of takeout was observed in chromosomal females expressing fru in fat body (lane 4). Females expressing both fru and dsxSWE (lane 8) have takeout expression levels indistinguishable from their male siblings (lane 7). takeout expression in wildtype males and females is shown in lane 1 and 2 (Modified with permission from Dauwalder et al. Genes & Dev., Nov 2002; 16: 2879 – 2892, Copyright 2002, Cold Spring Harbor Laboratory Press).
Recent micro-array based genomic studies that examined the expression of genes that are regulated by the sex determination hierarchy in the heads of flies have identified new modes of DSX regulated gene expression [45]. These studies suggest that the model of regulation that is seen in yolk protein genes and takeout, namely that one form activates and the other represses, is true only for a subset of dsx regulated transcripts. For others, expression was lower in both sexes when dsx was mutated, indicating that they are usually activated by both DSX-M and DSX-F. Another class was higher in both mutants, indicating that both DSX forms usually repress these transcripts. The reason why these genes were found to be expressed at different levels in the two sexes in the first place was that DSX-F appeared to both activate and repress to a greater extent. This may be due to the fact that DSX-F interacts with another protein encoded by intersex [60], which could make it a more potent activator and repressor. In addition, there was a class of transcripts where DSX was only required in one sex. The same study also identified genes that were regulated by FRUM. When whole heads and dissected brains were compared, it was discovered that a majority of the identified DSX and FRUM targets was expressed outside of the nervous system. These genes are most likely expressed in the fat body, or perhaps in glial cells. These findings indicate that there may be a fairly large number of sex specific transcripts in the fat body, supporting earlier findings about its sex specific function. Further studies will be required to determine the role of individual genes and whether/how they contribute to sex specific behaviors.
Since FRUM expression has so far not been observed in fat body [5,6,11], the finding that a significant number of its transcripts are regulated by fru poses the question of how this regulation occurs. Unless fru levels in the fat body were below detection threshold, FRUM probably acts indirectly, perhaps by influencing the generation of a circulating signal, or via other effects mediated by neuronal activity of FRUM expressing cells. Very few FRUM targets were identified in the nervous system, possibly because they are expressed only in small subsets of FRUM expressing cells and therefore may not have been detected under the stringent criteria of the screen [45]. One of the identified FRUM targets, dpr (defective proboscis extension response), was found to affect courtship. Mutant males showed reduced courtship latency and reduced time to copulation. Interestingly, dpr was expressed in ascending median bundle neurons that express FRUM and in earlier studies had been shown to regulate the timing of courtship [30].
Not only is there mounting evidence for the crucial role of the fat body, but in addition, a recent study by Grosjean et al. [61] has shown a contribution of glial cells in the brain. A mutation in the gene “genderblind” which is expressed in CNS glial cells, causes males to become non-discriminatory and court females and males alike. This is most likely due to their overreaction to and improper processing of chemosensory cues, since they do not court desat1 mutant males which produce very small amounts of sex specific pheromones. However, they do court desat1 males that have been “painted” with 7-tricosene, a pheromone that is thought to normally prevent male-male courtship. genderblind codes for a transporter that regulates extracellular glutamate, an indication that glutamatergic neurons are involved in the processing of pheromone detection.
Taken together our current knowledge of male courtship behavior shows an intricate network of neuronal circuits that are set up under the control of both the fruitless and doublesex genes and that together confer the neuronal competence for the behavior (also discussed in [62]). However, perhaps surprisingly, efficient and normal courtship is dependent on additional input from non-neuronal tissues, such as the fat body and glial cells. Diffusible sex specific factors secreted from the fat body may play an important role in this regulation, suggesting that sexually dimorphic characters in Drosophila result from the interaction of sex-determining genes and endocrine factors.
ACKNOWLEDGEMENTS
Thanks to William Mattox for critical reading of the manuscript, to Tony Dornan and Stephen Goodwin for their figure showing fru expression in the brain, and to Daisuke Yamamoto for UAS-fru flies. This work was supported by NSF grant IOB 0416476 to B.D.
REFERENCES
- 1.Greenspan RJ, Ferveur JF. Courtship in Drosophila. Annu. Rev. Genet. 2000;34:205–232. doi: 10.1146/annurev.genet.34.1.205. [DOI] [PubMed] [Google Scholar]
- 2.Hall JC. The mating of a fly. Science. 1994;264:1702–1714. doi: 10.1126/science.8209251. [DOI] [PubMed] [Google Scholar]
- 3.Cline TW, Meyer BJ. Vive la difference: males vs females in flies vs worms. Annu. Rev. Genet. 1996;30:637–702. doi: 10.1146/annurev.genet.30.1.637. [DOI] [PubMed] [Google Scholar]
- 4.Christiansen AE, Keisman EL, Ahmad SM, Baker BS. Sex comes in from the cold: the integration of sex and pattern. Trends. Genet. 2002;18:510–516. doi: 10.1016/s0168-9525(02)02769-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee G, Foss M, Goodwin SF, Carlo T, Taylor BJ, Hall JC. Spatial, temporal, and sexually dimorphic expression patterns of the fruitless gene in the Drosophila central nervous system. J. Neurobiol. 2000;43:404–426. doi: 10.1002/1097-4695(20000615)43:4<404::aid-neu8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 6.Usui-Aoki K, Ito H, Ui-Tei K, Takahashi K, Lukacsovich T, Awano W, Nakata H, Piao ZF, Nilsson EE, Tomida J, Yamamoto D. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nat. Cell Biol. 2000;2:500–506. doi: 10.1038/35019537. [DOI] [PubMed] [Google Scholar]
- 7.McRobert SP, Tompkins L. The effect of transformer, doublesex and intersex mutations on the sexual behavior of Drosophila melanogaster. Genetics. 1985;111:89–96. doi: 10.1093/genetics/111.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor BJ, Villella A, Ryner LC, Baker BS, Hall JC. Behavioral and neurobiological implications of sex-determining factors in Drosophila. Dev. Genet. 1994;15:275–296. doi: 10.1002/dvg.1020150309. [DOI] [PubMed] [Google Scholar]
- 9.Villella A, Hall JC. Courtship anomalies caused by doublesex mutations in Drosophila melanogaster. Genetics. 1996;143:331–344. doi: 10.1093/genetics/143.1.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gailey DA, Hall JC. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics. 1989;121:773–785. doi: 10.1093/genetics/121.4.773. [published erratum appears in Genetics 1989,122:465] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodwin SF, Taylor BJ, Villella A, Foss M, Ryner LC, Baker BS, Hall JC. Aberrant splicing and altered spatial expression patterns in fruitless mutants of Drosophila melanogaster. Genetics. 2000;154:725–745. doi: 10.1093/genetics/154.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito H, Fujitani K, Usui K, Shimizu-Nishikawa K, Tanaka S, Yamamoto D. Sexual orientation in Drosophila is altered by the satori mutation in the sex-determination gene fruitless that encodes a zinc finger protein with a BTB domain. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9687–9692. doi: 10.1073/pnas.93.18.9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ryner LC, Goodwin SF, Castrillon DH, Anand A, Villella A, Baker BS, Hall JC, Taylor BJ, Wasserman SA. Control of male sexual behavior and sexual orientation in Drosophila by the fruitless gene. Cell. 1996;87:1079–1089. doi: 10.1016/s0092-8674(00)81802-4. [DOI] [PubMed] [Google Scholar]
- 14.Villella A, Gailey DA, Berwald B, Ohshima S, Barnes PT, Hall JC. Extended reproductive roles of the fruitless gene in Drosophila melanogaster revealed by behavioral analysis of new fru mutants. Genetics. 1997;147:1107–1130. doi: 10.1093/genetics/147.3.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baker BS, Taylor BJ, Hall JC. Are complex behaviors specified by dedicated regulatory genes? Reasoning from Drosophila. Cell. 2001;105:13–24. doi: 10.1016/s0092-8674(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 16.Billeter JC, Rideout EJ, Dornan AJ, Goodwin SF. Control of male sexual behavior in Drosophila by the sex determination pathway. Curr. Biol. 2006;16:R766–776. doi: 10.1016/j.cub.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Manoli DS, Meissner GW, Baker BS. Blueprints for behavior: genetic specification of neural circuitry for innate behaviors. Trends Neurosci. 2006;29:444–451. doi: 10.1016/j.tins.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto D. The neural and genetic substrates of sexual behavior in Drosophila. Adv. Genet. 2007;59:39–66. doi: 10.1016/S0065-2660(07)59002-4. [DOI] [PubMed] [Google Scholar]
- 19.Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436:395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- 20.Stockinger P, Kvitsiani D, Rotkopf S, Tirian L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Lee G, Hall JC. A newly uncovered phenotype associated with the fruitless gene of Drosophila melanogaster: aggression-like head interactions between mutant males. Behav. Genet. 2000;30:263–275. doi: 10.1023/a:1026541215546. [DOI] [PubMed] [Google Scholar]
- 22.Vrontou E, Nilsen SP, Demir E, Kravitz EA, Dickson BJ. fruitless regulates aggression and dominance in Drosophila. Nat. Neurosci. 2006;9:1469–1471. doi: 10.1038/nn1809. [DOI] [PubMed] [Google Scholar]
- 23.Nilsen SP, Chan YB, Huber R, Kravitz EA. Gender-selective patterns of aggressive behavior in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 2004;101:12342–12347. doi: 10.1073/pnas.0404693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anand A, Villella A, Ryner LC, Carlo T, Goodwin SF, Song HJ, Gailey DA, Morales A, Hall JC, Baker BS, Taylor BJ. Molecular Genetic Dissection of the Sex-Specific and Vital Functions of the Drosophila melanogaster Sex Determination Gene fruitless. Genetics. 2001;58:1569–1595. doi: 10.1093/genetics/158.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrichs V, Ryner LC, Baker BS. Regulation of sex-specific selection of fruitless 5' splice sites by transformer and transformer-2. Mol. Cell. Biol. 1998;18:450–458. doi: 10.1128/mcb.18.1.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferri SL, Bohm RA, Lincicome HE, Hall JC, Villella A. fruitless Gene products truncated of their male-like qualities promote neural and behavioral maleness in Drosophila if these proteins are produced in the right places at the right times. J. Neurogenet. 2008;22:17–55. doi: 10.1080/01677060701671947. [DOI] [PubMed] [Google Scholar]
- 27.Billeter JC, Villella A, Allendorfer JB, Dornan AJ, Richardson M, Gailey DA, Goodwin SF. Isoform-specific control of male neuronal differentiation and behavior in Drosophila by the fruitless gene. Curr. Biol. 2006;16:1063–1076. doi: 10.1016/j.cub.2006.04.039. [DOI] [PubMed] [Google Scholar]
- 28.Dornan AJ, Gailey DA, Goodwin SF. GAL4 enhancer trap targeting of the Drosophila sex determination gene fruitless. Genesis. 2005;42:236–246. doi: 10.1002/gene.20143. [DOI] [PubMed] [Google Scholar]
- 29.Kimura K, Ote M, Tazawa T, Yamamoto D. Fruitless specifies sexually dimorphic neural circuitry in the Drosophila brain. Nature. 2005;438:229–233. doi: 10.1038/nature04229. [DOI] [PubMed] [Google Scholar]
- 30.Manoli DS, Baker BS. Median bundle neurons coordinate behaviours during Drosophila male courtship. Nature. 2004;430:564–569. doi: 10.1038/nature02713. [DOI] [PubMed] [Google Scholar]
- 31.Kondoh Y, Kaneshiro KY, Kimura K, Yamamoto D. Evolution of sexual dimorphism in the olfactory brain of Hawaiian Drosophila. Proc. Biol. Sci. 2003;270:1005–1013. doi: 10.1098/rspb.2003.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ejima A, Smith BP, Lucas C, van der Goes van Naters W, Miller CJ, Carlson JR, Levine JD, Griffith LC. Generalization of courtship learning in Drosophila is mediated by cis-vaccenyl acetate. Curr. Biol. 2007;17:599–605. doi: 10.1016/j.cub.2007.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 34.Datta SR, Vasconcelos ML, Ruta V, Luo S, Wong A, Demir E, Flores J, Balonze K, Dickson BJ, Axel R. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 35.Chan YB, Kravitz EA. Specific subgroups of FruM neurons control sexually dimorphic patterns of aggression in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 2007;104:19577–19582. doi: 10.1073/pnas.0709803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hall JC. Control of male reproductive behavior by the central nervous system of Drosophila: dissection of a courtship pathway by genetic mosaics. Genetics. 1979;92:437–457. doi: 10.1093/genetics/92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rideout EJ, Billeter JC, Goodwin SF. The sex-determination genes fruitless and doublesex specify a neural substrate required for courtship song. Curr. Biol. 2007;17:1473–1478. doi: 10.1016/j.cub.2007.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor BJ, Truman JW. Commitment of abdominal neuroblasts in Drosophila to a male or female fate is dependent on genes of the sex-determining hierarchy. Development. 1992;114:625–642. doi: 10.1242/dev.114.3.625. [DOI] [PubMed] [Google Scholar]
- 39.Clyne JD, Miesenbock G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133:354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 40.Shirangi TR, Taylor BJ, McKeown M. A double-switch system regulates male courtship behavior in male and female Drosophila melanogaster. Nat. Genet. 2006;38:1435–1439. doi: 10.1038/ng1908. [DOI] [PubMed] [Google Scholar]
- 41.Fujii S, Amrein H. Genes expressed in the Drosophila head reveal a role for fat cells in sex-specific physiology. EMBO J. 2002;21:5353–5363. doi: 10.1093/emboj/cdf556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arbeitman MN, Fleming AA, Siegal ML, Null BH, Baker BS. A genomic analysis of Drosophila somatic sexual differentiation and its regulation. Development. 2004;131:2007–2021. doi: 10.1242/dev.01077. [DOI] [PubMed] [Google Scholar]
- 43.Arbeitman MN, Furlong EE, Imam F, Johnson E, Null BH, Baker BS, Krasnow MA, Scott MP, Davis RW, White KP. Gene expression during the life cycle of Drosophila melanogaster. Science. 2002;297:2270–2275. doi: 10.1126/science.1072152. [DOI] [PubMed] [Google Scholar]
- 44.Dauwalder B, Tsujimoto S, Moss J, Mattox W. The Drosophila takeout gene is regulated by the somatic sex-determination pathway and affects male courtship behavior. Genes Dev. 2002;16:2879–2892. doi: 10.1101/gad.1010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goldman TD, Arbeitman MN. Genomic and functional studies of Drosophila sex hierarchy regulated gene expression in adult head and nervous system tissues. PLoS Genet. 2007;3:e216. doi: 10.1371/journal.pgen.0030216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bownes M, Hames BD. Accumulation and degradation of three major yolk proteins in Drosophila melanogaster. J. Exp. Zool. 1977;200:149–156. doi: 10.1002/jez.1402000118. [DOI] [PubMed] [Google Scholar]
- 47.Haunerland NH. Insect storage proteins: gene families and receptors. Insect Biochem. Mo. Biol. 1996;26:755–765. doi: 10.1016/s0965-1748(96)00035-5. [DOI] [PubMed] [Google Scholar]
- 48.Meister M, Lemaitre B, Hoffmann JA. Antimicrobial peptide defense in Drosophila. Bioessays. 1997;19:1019–1026. doi: 10.1002/bies.950191112. [DOI] [PubMed] [Google Scholar]
- 49.Bownes M. The regulation of the yolk protein genes, a family of sex differentiation genes in Drosophila melanogaster. Bioessays. 1994;16:745–752. doi: 10.1002/bies.950161009. [DOI] [PubMed] [Google Scholar]
- 50.Lazareva AA, Roman G, Mattox W, Hardin PE, Dauwalder B. A role for the adult fat body in Drosophila male courtship behavior. PLoS Genet. 2007;3:e16. doi: 10.1371/journal.pgen.0030016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carney GE. A rapid genome-wide response to Drosophila melanogaster social interactions. BMC Genomics. 2007;8:288. doi: 10.1186/1471-2164-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarov-Blat L, So WV, Liu L, Rosbash M. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell. 2000;101:647–656. doi: 10.1016/s0092-8674(00)80876-4. [DOI] [PubMed] [Google Scholar]
- 53.So WV, Sarov-Blat L, Kotarski CK, McDonald MJ, Allada R, Rosbash M. takeout, a novel Drosophila gene under circadian clock transcriptional regulation. Mol. Cell. Biol. 2000;20:6935–6944. doi: 10.1128/mcb.20.18.6935-6944.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Burtis KC, Coschigano KT, Baker BS, Wensink PC. The doublesex proteins of Drosophila melanogaster bind directly to a sex-specific yolk protein gene enhancer. Embo J. 1991;10:2577–2582. doi: 10.1002/j.1460-2075.1991.tb07798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho S, Wensink PC. Purification and physical properties of the male and female double sex proteins of Drosophila. Proc. Natl. Acad. Sci. U.S.A. 1996;93:2043–2047. doi: 10.1073/pnas.93.5.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coschigano KT, Wensink PC. Sex-specific transcriptional regulation by the male and female doublesex proteins of Drosophila. Genes Dev. 1993;7:42–54. doi: 10.1101/gad.7.1.42. [DOI] [PubMed] [Google Scholar]
- 57.An W, Wensink PC. Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev. 1995;9:256–266. doi: 10.1101/gad.9.2.256. [DOI] [PubMed] [Google Scholar]
- 58.Burtis KC. The regulation of sex determination and sexually dimorphic differentiation in Drosophila. Curr. Opin. Cell Biol. 1993;5:1006–1014. doi: 10.1016/0955-0674(93)90085-5. [DOI] [PubMed] [Google Scholar]
- 59.Erdman SE, Chen HJ, Burtis KC. Functional and genetic characterization of the oligomerization and DNA binding properties of the Drosophila doublesex proteins. Genetics. 1996;144:1639–1652. doi: 10.1093/genetics/144.4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garrett-Engele CM, Siegal ML, Manoli DS, Williams BC, Li H, Baker BS. intersex, a gene required for female sexual development in Drosophila, is expressed in both sexes and functions together with doublesex to regulate terminal differentiation. Development. 2002;129:4661–4675. doi: 10.1242/dev.129.20.4661. [DOI] [PubMed] [Google Scholar]
- 61.Grosjean Y, Grillet M, Augustin H, Ferveur JF, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat. Neurosci. 2008;11:54–61. doi: 10.1038/nn2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shirangi TR, McKeown M. Sex in flies: what 'body--mind' dichotomy? Dev. Biol. 2007;306:10–19. doi: 10.1016/j.ydbio.2007.03.022. [DOI] [PubMed] [Google Scholar]