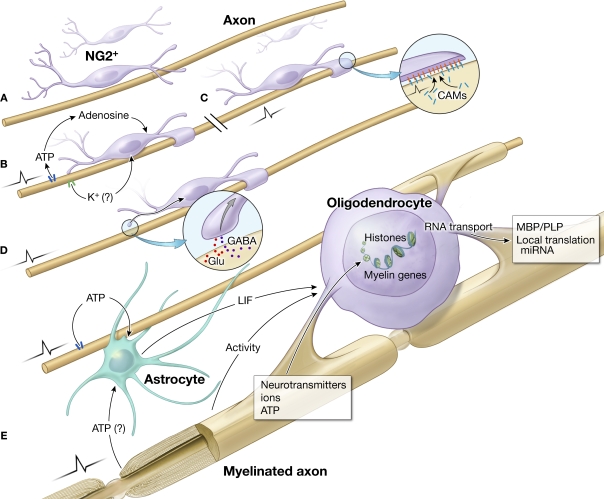
Figure 1.
Impulse activity in axons regulates oligodendrocyte development and myelination at several stages and via different signals. (A) Immature OPCs (NG2+ cells) in white matter on an electrically silent unmyelinated axon. Such cells persist in significant numbers in the adult brain. (B) Electrical activity causes ATP release from axons, which generates adenosine that stimulates differentiation of NG2 cells to a mature oligodendrocyte, and promotes myelination (Stevens et al., 2002). K+ is released from electrically active axons. Blocking K+ channels in oligodendrocytes in culture has been shown to regulate oligodendrocyte proliferation and lineage progression (Ghiani et al., 1999). (C) Electrical activity can also alter the expression of cell adhesion molecules on the axon that are involved in initiating myelination (Itoh et al., 1995, 1997). This has been shown to regulate myelination by Schwann cells in the PNS, but the same molecule (L1-CAM) is involved in myelination by oligodendrocytes (Barbin et al., 2004). (D) The release of the neurotransmitters Glu (glutamate) or GABA from synapses formed on NG2 cells (Kukley et al., 2007), could provide another mechanism to regulate myelination in response to functional activity. (E) After NG2 cells differentiate into oligodendrocytes, ATP released from axons firing action potentials stimulates the synthesis and release of the cytokine LIF from astrocytes, which promotes myelination (Ishibashi et al., 2006). Myelination during development and postnatally may be regulated by several other unidentified activity-dependent signaling molecules affecting development of oligodendrocytes and myelin formation. Electrical activity in axons, via the release of neurotransmitters, ions and ATP may influence gene expression in oligodendrocytes by histone modification, RNA transport, local translation and regulate mRNA stability and translation by miRNAs.