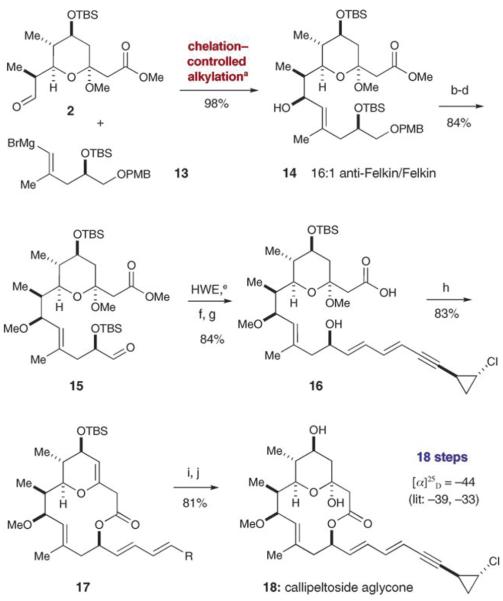
Scheme 3.
Coupling the iodoalcohol and tetrahydropyran: a) MgBr2·Et2O, CH2Cl2, −78°C; b) MeOTf, 2,6-DTBP, CH2Cl2; c) DDQ, CH2Cl2, pH 7 buffer; d) SO3·pyridine, Et3N, CH2Cl2, DMSO; e) LiHMDS, then 5, THF, −78°C; f) TBAF, THF, 0°C; g) Ba(OH)2·8 H2O, MeOH; h) Yamaguchi: 2,4,6-Cl3C6H2COCl, iPr2EtN, THF, DMAP, toluene, 60°C; i) PPh3·HBr, H2O, CH2Cl2. j) TFA, THF, H2O. DMAP=4-dimethylaminopyridine, 2,6-DTBP=2,6-di-tert-butylpyridine, HMDS=hexamethyldisilazane, TBAF=tetrabutylammonium fluoride, TFA=trifluoroacetic acid.