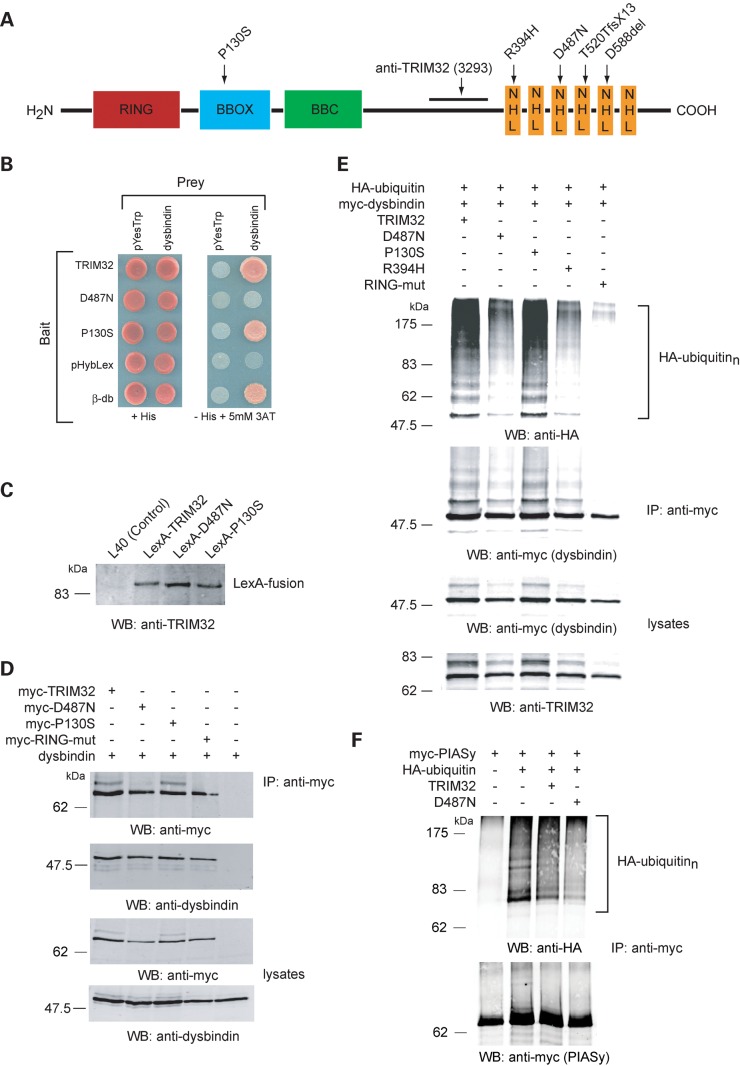
Figure 1.
Dysbindin interacts with TRIM32 in yeast and in vivo. TRIM32 and P130S interact with dysbindin in the Y2H system. (A) Schematic representation of the domain organization of TRIM32 and location of the disease-associated mutations and the region used for polyclonal rabbit anti-sera generation. (B) TRIM32, D487N and P130S bait strains were co-transformed with empty prey plasmid (pYesTrp2) or dysbindin prey plasmid. The double transformants were tested for histidine auxotrophy. The previously characterized β-dystrobrevin (β-db) bait is used as a positive control for the dysbindin interaction. (C) Bait strain protein lysates were western blotted with the TRIM32 3293 antibody, showing all three DNA-binding LexA-fusions are synthesized at comparable levels in yeast. (D) TRIM32 and dysbindin interact in mammalian cells. HEK293T cells were transfected with dysbindin and myc-TRIM32, myc-D487N, myc-P130S, myc-RING-mut. Dysbindin co-immunoprecipitated only in the presence of myc-TRIM32 (or any of the mutants), indicating they form a complex in transfected cells. (E) Dysbindin is polyubiquitinated by TRIM32. Myc-dysbindin was immunoprecipitated from HEK293T cells expressing HA-ubiquitin and either TRIM32, D487N, P130S, R394H or RING-mut. Immunoprecipitated dysbindin was probed for the presence of conjugated HA-ubiquitin by western blot. A high molecular weight smear is seen on the HA blot indicative of polyubiquitination. As expected, the RING mutant is unable to ubiquitinate dysbindin. Interestingly, D487N and R394H have impaired ubiquitin ligase activity towards dysbindin, exemplified by the weaker smearing on the HA blot. (F) Effect of TRIM32 on the ubiquitination of PIASy. The ubiquitination of PIASy was assessed under identical condition to those for dysbindin as shown in (E). Co-expression of TRIM32 or TRIM32–D487N had no apparent effect upon the ubiquitination of PIASy. Note that PIASy is modified by endogenous ubiquitin ligases when expressed in HEK293T cells.