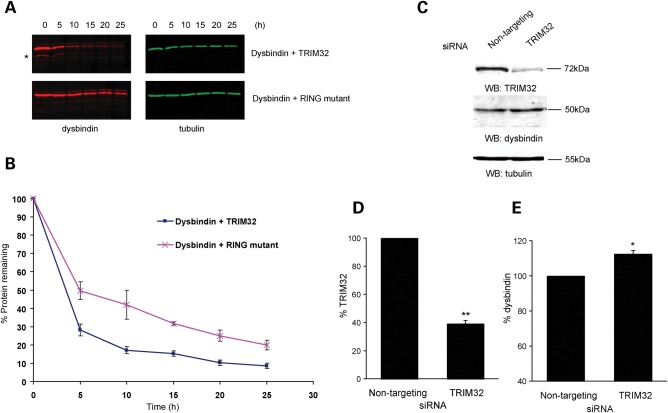
Figure 4.
TRIM32 targets dysbindin for degradation. (A) COS-7 cells were transfected with dysbindin (red) and TRIM32 (green), or dysbindin and the RING mutant (RING-mut) (all untagged in pCIneo). Protein synthesis was blocked by cycloheximide treatment (50 µg/ml) and samples taken every 5 h. The levels of dysbindin in lysates was analysed by quantitative western and plotted graphically (B). The presence of TRIM32 destabilizes dysbindin, increasing its turnover relative to co-expression the RING-mut. Additional bands that might be possible breakdown products of dysbindin can be seen below the main dysbindin band in the presence of TRIM32, but not the RING-mut (asterisk in A). The error bars show the standard error of the mean from three independent experiments. (C) Knockdown of TRIM32 in C2C12s by siRNA increases dysbindin levels. C2C12 cells were transfected on consecutive days with either control siRNA (non-targeting pool) or TRIM32 siRNA. Seventy-two hours after the first transfection lysates were made and levels of TRIM32, dysbindin and tubulin were analysed by quantitative western blot (WB). TRIM32 and dysbindin levels were normalized to tubulin and plotted as a chart (D). The error bars show the standard error of the mean from three independent experiments. The TRIM32-specific J-12 duplex achieved ∼60% knockdown relative to the control siRNA (**P < 0.01 in a one-sample two-tailed t-test). (E) Knockdown of TRIM32 results in a significant increase in dysbindin levels relative to control (*P < 0.05 in a one-sample two-tailed t-test).