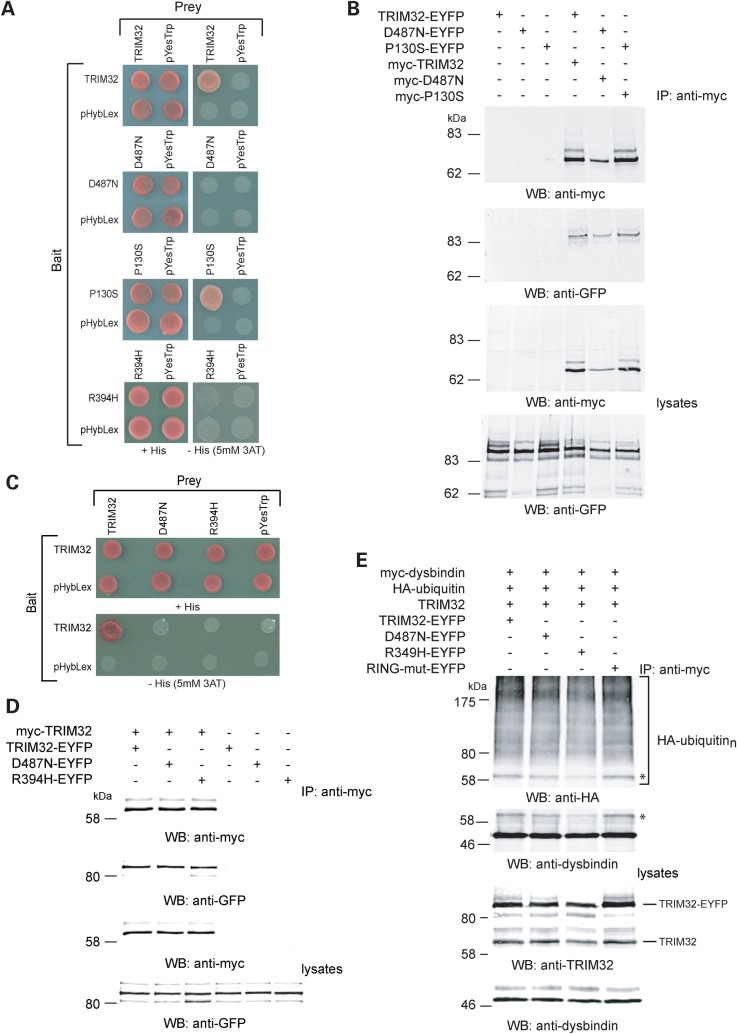
Figure 5.
Self-association properties of TRIM32 and mutants. (A) Y2H-mediated analysis of TRIM32 self-association. Co-transformants containing TRIM32, D487N, P130S, R394H bait and prey plasmids or empty bait, and prey plasmids were tested for histidine auxotrophy on media lacking histidine containing 5 mm 3-AT. Only wild-type TRIM32 and P130S could self-associate in yeast. In each case, selective and non-selective media are compared. (B) Wild-type TRIM32, D487N and P130S self-associate in mammalian cells. HEK293T cells were transfected with myc-tagged TRIM32, D487N or P130S and their EYFP-tagged counterparts. EYFP-tagged proteins are only immunoprecipitated in the presence of the myc-tagged protein, indicating that the myc-tagged and EYFP-tagged proteins interact. (C) The LGMD2H/STM mutants failed to interact with wild-type TRIM32 in yeast. Bait and preys were co-transformed into yeast as indicated. TRIM32 is able to self-associate, but failed to interact with either of the LGMD2H/STM mutants. In each case, selective and non-selective media are compared. (D) Co-immunoprecipitation of wild-type TRIM32 with the LGMD2H/STM mutants, D487N and R394H. HEK293T cells were transfected with the constructed as indicated. Proteins were immunoprecipitated from the cell extracts using the anti-myc antibody. The anti-GFP antibody was used to detect the co-immunoprecipitated EYFP-tagged TRIM32. In each case, TRIM32 and the each of the mutants co-immunoprecipitated with myc-tagged wild-type TRIM32. The EYFP-tagged proteins did not immunoprecipitate in the absence of myc-tagged wild-type TRIM32 demonstrating that non-specific binding to the beads was not a confounding factor in this experiment. (E) Dysbindin ubiquitination in cells co-expressing wild-type and mutant TRIM32s. Ubiquitination assays were performed as described above (Fig. 1E). Myc-tagged dysbindin was immunoprecipitated from cells expressing the designated combination of plasmids. In each case, wild-type TRIM32 was co-expressed with either 0.5 µg of TRIM32–EYFP, D487N–EYFP, R394H–EYFP or RING-mut–EYFP. Dysbindin ubiquitination was assessed with the anti-HA antibody as described above. In each reaction, dysbindin is clearly polyubiquitinated by each combination of TRIM32 and mutant. However in extracts from cells expressing myc-TRIM32 and R394H–EYFP, we consistently found a reduction in the levels of the monoubiquitinated form of dysbindin (asterisk). This change was also detected with the anti-dysbindin antibody (asterisk). Inspection of the lysates revealed that similar levels of myc-TRIM32, dysbindin and the EYFP-tagged mutants were produced in each transfection.