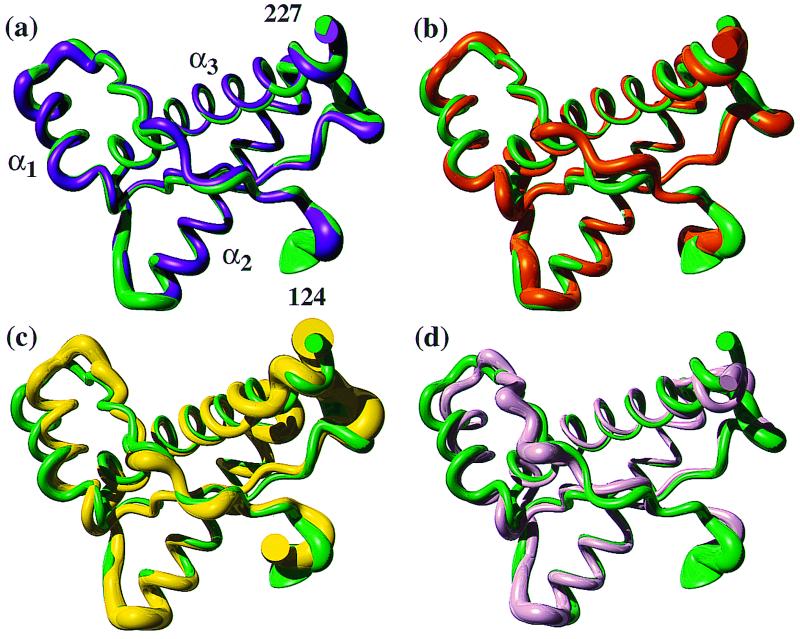
Figure 3.
(a) Superposition of the mean NMR structures of the polypeptide segment 124–227 in bPrP(23–230) (violet) and bPrP(121–230) (green). A spline function was drawn through the Cα positions. The variable radius of the cylindrical rods is proportional to the mean global backbone displacement per residue (43), as evaluated after superposition for best fit of the atoms N, Cα, and C′ of the residues 125–227 in the two bundles of 20 energy-minimized conformers used to represent the solution structures (29). (b–d) Superposition of the segment 125–227 in bPrP(121–230) (green) with the corresponding residues in hPrP(121–230) (b; orange), mPrP(121–231) (c; yellow), and shPrP(90–231) (d; pink), respectively.