Abstract
Necrotizing enterocolitis (NEC) remains a leading cause of morbidity and mortality in preterm infants. Although its pathogenesis is poorly understood, inappropriate apoptosis of the mucosal epithelia has been implicated. Recent clinical trials have shown probiotics may reduce the incidence of NEC, and probiotics have been shown to suppress intestinal epithelial apoptosis in cultured cells. However, little is known about their mechanism of action in the developing intestine in vivo. Here, we confirm that the probiotic Lactobacillus rhamnosus GG (LGG) reduces chemically induced intestinal epithelial apoptosis in vitro. Furthermore, we report for the first time that LGG administered orally to live animals can reduce chemically induced epithelial apoptosis ex vivo, as measured by staining for active caspase 3 and TUNEL. Utilizing cDNA microarray analysis from the intestine of live, orally inoculated mice, we show that LGG upregulates of a battery of genes with known and likely cytoprotective effects. These studies indicate that probiotics such as LGG may augment intestinal host defenses in the developing intestine by stimulating anti-apoptotic and cytoprotective responses. Since apoptosis may be a precursor to NEC, understanding the mechanism behind probiotic modulation of apoptotic pathways may allow for development of more specifically targeted therapies or preventive strategies in the future.
Keywords: Necrotizing enterocolitis, probiotics, Lactobacillus, apoptosis, intestinal epithelia
Necrotizing enterocolitis (NEC) causes significant neonatal morbidity and mortality in very low birthweight (VLBW) infants (1). Despite advances in the supportive care of premature infants, little progress has been made in the prevention or treatment of NEC. Its etiology has not been fully elucidated, but is likely multifactorial, involving immaturity of intestinal host defenses and abnormal bacterial colonization (1-4). Recently, aberrant or excessive apoptosis has been increasingly recognized as either an initiating event or necessary step in the pathogenesis of NEC (1, 5-7). Cells commonly undergo apoptosis in response to injurious stimuli (microbial, hypoxic, or chemical) (8), allowing dismantling of damaged cells without release of cellular contents and aggravation of tissue injury. However, excessive or inappropriate apoptosis may cause tissue injury and clinical consequences. Histopathologic evaluation of resected specimens from infants with surgical NEC reveal apoptosis as early events in the disease process (9, 10), and animal models of NEC show that epithelial apoptosis precedes the gross bowel necrosis characteristic of the disease (5). Furthermore, inhibiting apoptotic pathways reduces the development of experimental NEC in rats, implying that apoptosis plays an early and important role in the pathogenesis of NEC, and modulation of this process could be exploited for therapeutic benefit (5-7).
VLBW infants at greatest risk for developing NEC are also at greatest risk of developing abnormal intestinal bacterial colonization due to exposure to nosocomial flora and frequent antibiotic administration (11). In healthy term breastfed neonates, commensal bacteria such as Bifidobacteria and other facultative anaerobes colonize the stool after the first 2 weeks of life (12). In contrast, VLBW and hospitalized infants exhibit delayed Bifidobacterium stool colonization and tend to colonize with a predominance of coliforms, other gram negative organisms, and Streptococcus (12). Abnormal bacterial colonization of the upper gastrointestinal tract with Enterobacteriaciae (13) has been reported in VLBW infants and early stool colonization with Clostridium perfringens has been associated with later development of NEC (14). Thus, inappropriate bacterial colonization may result in a “dysbiotic” intestinal flora that may inflict or contribute to injury of the immature gut and potentially predispose to NEC.
Probiotics are defined as ‘living micro-organisms, which upon ingestion in sufficient numbers, exert health benefits beyond basic nutrition’ (15). Probiotics can improve intestinal host defenses not only by normalizing intestinal colonization patterns but also by directly affecting intestinal epithelial function. Studies have shown commensal bacteria regulate many intestinal defenses including barrier function, mucin and IgA secretion, inflammation, and homeostatic processes such as proliferation and apoptosis (16-20). In animal models, probiotics can reduce the severity (21) and incidence (22, 23) of experimental NEC. Probiotics may be effective clinically in the prevention of NEC, and bacteria studied in clinical trials include Lactobacillus, Bifidiobacterium, and Streptococcus thermophilus (24-27). Recent in vitro studies indicate that the probiotic Lactobacillus rhamnosus GG (LGG) may be particularly effective in preventing cytokine-induced apoptosis in adult intestinal epithelial cells (20, 28).
Little is known about the effect of probiotics on inducible apoptosis in immature intestines. Cytokine-mediated apoptosis occurs via the extrinsic pathway and is stimulated by ligand/death receptor interactions. As the apoptosis observed in NEC may involve physical stresses (hypoxia) and exogenous signals (bacteria, food antigens), we sought to determine if LGG could suppress apoptosis stimulated by multiple pathways using the broad-spectrum pro-apoptotic agent staurosporine (STS). STS has been implicated to induce both caspase-dependent (intrinsic and extrinsic) and caspase-independent apoptotic pathways through both protein kinase C (PKC) dependent and independent mechanisms (29, 30). Here we report that the probiotic LGG reduces chemically induced (1μg/ml STS) intestinal epithelial apoptosis in vitro. Furthermore, we demonstrate that LGG decreases chemically induced apoptosis in the developing murine intestine. We modeled immature intestinal epithelia utilizing ex vivo organ culture of two week-old murine small intestines in which intestinal epithelial maturity resembles that of 24−28 week premature infants (31). Chemically induced intestinal epithelial cleaved caspase 3 and TUNEL staining was significantly reduced in mice prefed LGG as compared to carrier alone. Although both pathogenic and commensal bacteria can modulate gene regulatory responses in intestinal epithelia, we show that unlike the pathogenic bacterium Salmonella typhimurium, which induces genes primarily regulating proinflammatory responses(32), the probiotic LGG, induces genes primarily regulating cytoprotective responses in developing murine small intestines. These studies indicate that the probiotic LGG may exert beneficial effects on immature intestines in part by promoting anti-apoptotic and cytoprotective responses. Since apoptosis may be a precursor to NEC, understanding the mechanism behind probiotic modulation of apoptotic pathways may allow for development of more specifically targeted therapies or preventive strategies in the future.
MATERIALS AND METHODS
Cell and bacterial culture
Rat intestinal epithelial cells (IEC-6 from ATCC) were grown to confluence on cover slips and in culture plates as previously described(8). LGG (provided by ATCC) was prepared overnight in Lactobacillus broth at 37°C per ATCC guidelines. LGG cultures were washed, concentrated in DMEM, and applied to IEC-6 cells at 107 CFUs/ml or gavage fed to 2 week-old mice at 1010 CFUs/ml.
In vitro experiments
IEC-6 cells were pretreated in media with or without LGG for 2 hours in a 5%CO2 incubator at 37°C. Apoptosis was then induced using STS (1μg/ml) or DMSO control for 2 hours. Numbers of TUNEL positive nuclei were counted per high power field (HPF).
Animal care
All animal experiments were approved by the Institutional Animal Care and Use Committee at Emory University. C57BL/6J mice were bred in an animal facility at Emory University. 2 week-old mice were orally gavage fed 0.2ml of DMEM with or without LGG. After 4 hours, the mice were anesthetized in CO2 and then euthanized by cervical dislocation. Small intestines were isolated and immediately frozen in TRIzol (Invitrogen) for RNA isolation, fixed in 10% formalin for histologic staining, or utilized in ex vivo experiments.
Ex-vivo experiment
Intestines were surgically excised and opened lengthwise to expose the intestinal epithelia. Ex vivo intestines were then maintained in RPMI media in 24-well cell culture plates at 37°C and apoptosis induced with STS (1μg/ml) for 2 hours. Intestines were subsequently washed in PBS and immediately fixed in 10% formalin for histologic staining.
Histologic staining
After experimental treatment, cells on coverslips were washed and fixed in 4% Paraformaldehyde (Fischer) or intestinal tissue was fixed, paraffin embedded, and sections mounted on slides. TUNEL: Apoptotic cells were labeled by an InSitu Cell Death Detection Kit (Roche), using terminal deoxynucleotidyltransferase (TUNEL) according to manufacturer guidelines. In vitro counterstaining: Cells on coverslips were counterstained with 4,6-diamidino-2-phynylindole (DAPI, 1μg/ml). Cleaved caspase 3: Cells containing activated caspase 3 were detected using cleaved caspase 3 (Asp 175) antibody for immunohistochemistry (Cell Signaling) according to manufacturer's guidelines. Slides were treated with primary cleaved caspase 3 antibody overnight (1:50) followed by secondary antibody 1:500 for 1 hour (HRP-conjugated anti-rabbit IgG, Amersham Biosciences). Sigma Fast DAB tablet set (Sigma-Aldrich) was used as per manufacturers instructions. Ex vivo counterstaining: Slides were counterstained with hematoxylin. Quantification and statistical analysis: Number of positive cells was counted in a minimum of 10 HPFs. Statistical differences were analyzed by ANOVA.
cDNA microarray analysis
Total RNA isolated from homogenized intestinal tissue was subjected to DNase I digestion, purified (Qiagen kit), and quality verified by gel electrophoresis. Total sample RNA (30 μg) and universal mouse reference RNA (20 μg) was used to synthesize cDNA labeled with fluorlink Cy5 dCTP and Cy3 dCTP, respectively. Sample and reference cDNA were cohybridized onto the MEEBO mouse 38.5K gene chip (Vanderbilt MicroArray Shared Resources, Vanderbilt University). Washed and dried chips were scanned (GenePix 4100A), and images were captured and analyzed (GenePix Pro 5.0). Ratio of Cy5:Cy3 of untreated and treated samples was calculated to assess relative changes in gene expression. Fold induction of greater than 2.5 was considered positive.
Real-time quantitative reverse transcription-PCR (RT-PCR)
Total RNA was reverse transcribed from random hexamer primers using Multiscribe reverse transcriptase (Applied Biosystems, Foster City, CA). Real-time quantitative RT-PCR analysis (SYBR Green real-time PCR assay, Applied Biosystems) was performed on the reverse transcription cDNA products using primers for Dusp3, Areg, CDK2, Xrcc6, FoxF2, Errb2, Sox4, and Jak2 (Primer Express software, Applied Biosystems) and 18s rRNA (Taqman rRNA control reagents kit, Applied Biosystems) as described previously(33). Level of expression for a given gene was normalized to the 18s rRNA level of the same sample. Fold difference was the ratio of the normalized value of each experimental sample to that of control samples. Primer sequences are available upon request.
RESULTS
LGG reduces chemically- induced intestinal epithelial apoptosis in vitro
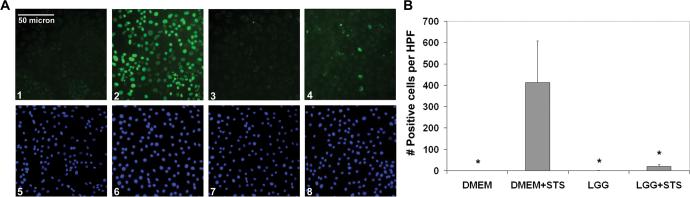
LGG has been shown to suppress cytokine-induced apoptosis in vitro in cultured epithelial cells (20). As mentioned previously, intestinal epithelial apoptosis observed to precede the gross histologic changes of NEC might be caused by multiple pathways not represented when using cytokines as a stimulant. Thus, to determine whether LGG could suppress apoptosis stimulated by multiple pathways, we used two intestinal epithelial cell lines, IEC-6 (rat) and Caco-2 (human) and the broad-spectrum pro-apoptotic agent, STS. Apoptosis was assayed by TUNEL staining. As expected, STS induced increased TUNEL staining in IEC-6 cells compared to media control (Fig. 1A, compare panels 1 and 2; and 1B, bars 1 and 2). Further, while LGG alone had no measurable effect on intestinal epithelial apoptosis, LGG pretreatment reduced STS-induced TUNEL positivity (Fig. 1A, compare panels 3 to 1; and B, bars 4 to 2). Essentially identical data were obtained in Caco-2 cells (data not shown). These results confirm that in vitro, LGG can reduce intestinal epithelial apoptosis stimulated with a broad-spectrum pro-apoptotic agent in both rodent and human cell lines.
Figure 1. LGG reduces chemically induced apoptosis in IEC-6 cells.
A, (1−4), TUNEL staining of IEC-6 cells treated with STS after preincubation with DMEM with (4) or without (2) LGG or treated with carrier control after preincubation with DMEM with (3) or without (1) LGG. (5−8), DAPI nuclear counterstaining of identical IEC-6 cells. B, Average number (mean ± SD) of TUNEL positive IEC-6 cells per HPF per treatment condition as indicated. Representative of 3 independent experiments. *P < 0.05 as compared to DMEM prefed, STS stimulated intestines (bar 2).
LGG decreases chemically induced epithelial apoptosis in the developing murine gut
To determine whether LGG has comparable effects on intestinal epithelial apoptosis in the developing gut, we modeled the developing intestine in two week-old preweaned mice (epithelial maturity of 24−28 premature human intestine) (31) and tested intestinal epithelial sensitivity to apoptosis ex vivo. We fed these immature mice media with or without LGG by oral gavage. Four hours later we surgically excised the small intestines for ex vivo chemical induction of apoptosis. Previous intestinal transit time experiments with aniline dye demonstrated that orally gavage fed material reaches the large intestine by 1 hour, and culture studies confirmed recovery of 1000-fold more live Lactobacillus in small intestines from LGG-fed mice at 4 hours post-gavage. As expected, control intestines isolated from mice fed media alone and stimulated ex vivo with STS demonstrated significantly increased epithelial TUNEL staining (especially at villus tips) compared to untreated intestines (Fig. 2A, compare panels 1 and 2; and 2B, first and second bars). Intestines harvested from mice treated with LGG exhibited significantly reduced epithelial apoptosis after ex vivo STS treatment (Fig. 2A, compare panels 2 and 4; and 2B, second and fourth bars). LGG alone had no effect (Fig. 2A, panels 1 and 3; and 2B, first and third bars). Next, we performed cleaved caspase 3 immunohistochemistry on the harvested intestines to test whether LGG reduces STS-induced apoptosis by inhibiting caspase 3 activation (final executioner caspase activity required for both intrinsic and extrinsic caspase-dependent apoptosis). As predicted, increased activated caspase 3 staining occurred after ex vivo STS treatment (Fig. 3A, panels 1 and 2; and 3B, first and second columns). Animals orally fed LGG before ex vivo experimental exposure exhibited significantly less caspase 3 activation as compared to those fed media alone (Fig. 3A, panels 2 and 4; and 3B, second and fourth columns). These studies indicate that LGG can reduce chemically induced apoptosis and caspase 3 activation in the developing murine intestine.
Figure 2. LGG reduces chemically induced intestinal epithelial apoptosis in the developing murine gut.
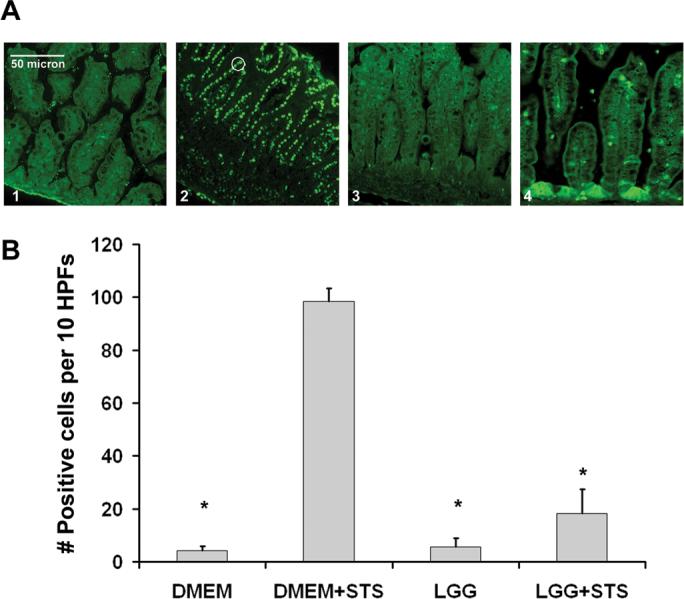
A, TUNEL staining of small intestines collected from 2 week-old mice prefed DMEM with (3) or without (1) LGG alone, or stimulated with STS ex vivo after prefeeding DMEM with (4) or without (2) LGG. Apoptotic cells noted by fluorescent green nuclei such as those circled (2). B, Average number (mean ± SD) of TUNEL positive intestinal epithelial cells per 10 HPFs per treatment condition as indicated (n=3 experiments). *P < 0.05 as compared to DMEM prefed, STS stimulated intestines (bar 2).
Figure 3. LGG reduces chemically induced intestinal epithelial caspase 3 cleavage in the developing murine gut.
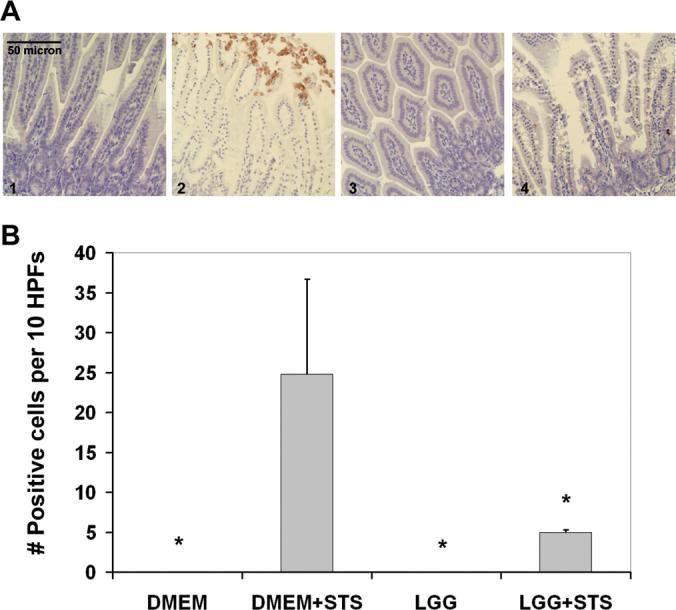
A, Cleaved caspase 3 staining of small intestines collected from 2 week-old mice prefed DMEM with (3) or without (1) LGG alone, or stimulated with STS ex vivo after prefeeding DMEM with (4) or without (2) LGG. B, Average number (mean ± SD) of TUNEL positive intestinal epithelial cells per 10 HPFs per treatment condition as indicated (n=3 experiments). *P < 0.05 as compared to DMEM prefed, STS stimulated intestines (bar 2).
LGG upregulates cytoprotective gene expression in the developing murine intestine
To further investigate the possible mechanism behind LGG reduction of experimental apoptosis in the immature intestine, we performed cDNA microarray analysis on intestines from our 2 week-old model of the developing murine gut. We collected small intestines from immature mice 4 hours after feeding LGG versus media alone. Based on previous intestinal transit time experiments, we expected that small intestinal epithelia would be exposed to the probiotic bacteria for 2−3 hours before harvesting. We have previously demonstrated that pathogenic bacteria such as Salmonella typhimurium can upregulate proinflammatory responses which in turn induce anti-apoptotic gene transcription (8). Here, we have analyzed the genes upregulated after oral feeding with LGG (Table 1, fold induction comparing bacteria fed to media fed mice). LGG induced upregulation of 185 genes and downregulation of 2400 genes in the small intestine of immature mice. 33 of the upregulated genes regulate putative cytoprotective processes (Table 1). In contrast to the primarily proinflammatory gene transcription pattern found after oral gavage of Salmonella typhimurium, genes upregulated after feeding LGG primarily regulate homeostatic processes such as cellular proliferation and migration and mitogen activated protein kinase (MAPK) pathways known to be important for growth, differentiation, and cytoprotection. Furthermore, unlike Salmonella typhimurium which induced anti-apoptotic gene upregulation through NF-κB and proinflammatory activation(8), LGG induced anti-apoptotic gene transcription without upregulation of proinflammatory genes. Selected genes (Table 1, italicized) upregulated by LGG as measured by cDNA microarray analysis were confirmed by quantitative real-time RT-PCR (Fig. 4). These results suggest that LGG may prevent apoptotic responses by upregulating anti-apoptotic and cytoprotective pathways in the developing gut.
Table 1. LGG upregulates anti-apoptotic and cytoprotective genes by cDNA microarray.
Selected upregulated genes in small intestines of LGG vs DMEM fed 2 week-old mice by cDNA microarray. Numbers represent average fold induction when comparing LGG to DMEM fed mice (n=3).
| CATEGORY | NAME | FOLD INDUCTION |
|---|---|---|
| Cell Proliferation/Migration | ||
| Guanine nucleotide binding protein, alpha q (Gnaq) | 26.1 | |
| LIM and senescent like antigen domains (Lims1/PINCH) | 7.2 | |
| Tight junction protein 2 (Tjp2/ZO-2) | 5.9 | |
| Carbonic anhydrase 8 (Car8) | 5.7 | |
| Frizzled homolog 1 (Fzd1/Fz-1) | 5.0 | |
| Amphiregulin (Areg) | 4.6 | |
| Cyclin-dependent kinase 2 (Cdk2) | 4.5 | |
| Sema domain, transmembrane domain (TM), and cytoplasmic domain, (semaphorin) 6D (Sema6D) | 4.4 | |
| Adenomatosis polyposis coli (APC) | 4.0 | |
| Choline phosphotransferase 1 (Chpt1) | 4.0 | |
| Homeodomain interacting protein kinase 1 (Hipk1) | 3.7 | |
| A kinase (PRKA) anchor protein (yotiao) 9 | 3.5 | |
| Ephrin B2 (Efnb2) | 3.5 | |
| Proliferating cell nuclear antigen (Pcna) | 3.4 | |
| Forkhead box F2 (FoxF2) | 3.4 | |
| Integrin beta 1 binding protein 1 (Itgb1bp1/Icap-1) | 3.3 | |
| Calpain 7 (clpn7) | 3.3 | |
| Microtubule-associated protein, RP/EB family, member 3 | 2.8 | |
| Zinc finger protein 326 | 2.8 | |
| Cell division cycle associated 7 (Cdca7) | 2.6 | |
| Kinase insert domain protein receptor (Kdr) | 2.6 | |
| MAP Kinase Related | ||
| Dual Specificity Phosphatase 3 (Dusp3/VHR) | 53.2 | |
| Guanine nucleotide binding protein, alpha inhibiting (Gnai3) | 13.7 | |
| Protein tyrosine phosphatase receptor, type E (Ptpre) | 4.8 | |
| Erbb2 interacting protein (Erbb2ip/Erbin) | 3.2 | |
| Neural precursor cell expressed, developmentally down-regulated gene 4-like (Nedd4l/Nedd4b) | 2.6 | |
| CREB/ATF bZIP transcription factor | 2.6 | |
| Anti-Apoptotic | ||
| Xray repair complementing defective repair (Xrcc6/Ku70) | 4.2 | |
| Zinc finger CCCH-type containing 15 (Zc3h15)/ Erythropoietin 4 immediate early response | 4.0 | |
| SRY-box containing gene 4 (Sox4) | 3.1 | |
| Pleckstrin homology domain interacting protein (PHIP) | 3.0 | |
| Apoptosis inhibitor 5 (Api5/AAC-11) | 2.8 | |
| Janus kinase 2 (Jak2) | 2.7 | |
Figure 4. LGG upregulates mRNA expression of anti-apoptotic and cytoprotective genes.
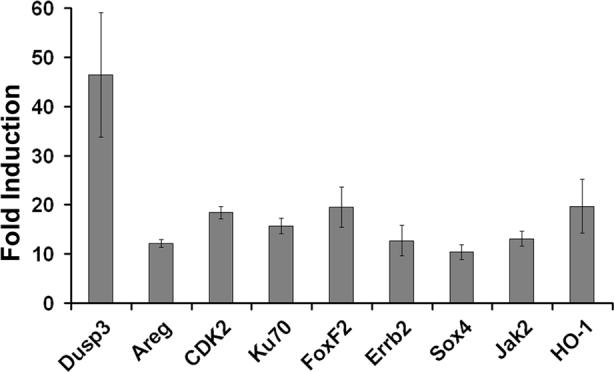
Quantitative real-time RT-PCR of genes expressed in the small intestine of LGG vs DMEM fed 2 week-old preweaned mice. Bars represent average fold induction (mean ± SD) when comparing mRNA levels in LGG to DMEM fed mice (n=3 experiments).
DISCUSSION
Probiotics have been shown to reduce the severity and incidence of both experimental NEC in animals (21-23) and clinical NEC in some small single center trials (24-27). Although these studies have stimulated increased interest worldwide in the potential use of probiotics to prevent NEC in VLBW infants, little work has been done to understand the mechanism by which probiotics exert a beneficial effect on the immature intestine (34). We show in this study that LGG, a well-studied probiotic bacterium that is also a member of the commensal flora, may protect immature intestines from apoptotic stimuli by upregulating a panel of anti-apoptotic/cytoprotective genes. Apoptosis is a form of programmed cell death that allows elimination of irreversibly damaged cells while preserving overall tissue function, and plays a vital role in normal developmental processes. However, overwhelming activation of apoptosis may result in tissue failure. Epithelial apoptosis has been described in the early stages of animal models of NEC where rat pups were subjected to repeated episodes of hypoxia, formula feeding and cold stress (5). Clinically, early NEC may also be due pro-apoptotic stresses in the gut due to hypoxia secondary to hypoperfusion, chemical injury from digestive products, or direct pro-apoptotic signaling from microbial stimuli.
In health, apoptosis is kept in check by basal expression of a spectrum of anti-apoptotic molecules such as the IAPs and anti-apoptotic members of the Bcl family that serve to inhibit the molecular apoptotic machinery. Additionally, these effectors are typically rapidly induced during inflammatory responses to counter increased exogenous stresses that accompany inflammation. Thus, transcriptional upregulation of anti-apoptotic effectors is considered characteristic of inflammatory signaling. It is very well-established by our laboratory and others that enteric pathogens elicit marked changes in epithelial gene regulation within 1−2 hours of exposure (32, 35-37), and similar experiments indicate that the adult gut can also respond to colonization with commensals (16, 32), though clearly the expression profiles are highly different. Our array data shows that probiotic bacteria are capable of stimulating new gene activation in the immature gut that is measurably different to that induced by classical enteric pathogens. These genes include not only known anti-apoptotic genes (Ku 70 (38), Sox4 (39), PHIP (40), Api5 (41), Jak2 (42)), but also candidate anti-apoptotic genes which regulate MAPK pathways (Dusp3 (43)). Multiple genes regulating cellular proliferation and migration as well as genes regulating MAPK pathways implicated in growth and development were also upregulated (Table 1). These genes may be cytoprotective by aiding in intestinal growth and healing, which may be especially important homeostatic processes in the developing gut.
How non-pathogenic bacteria activate epithelial signaling pathways is a topic of intense investigation. Classically, pathogens are monitored by cellular pattern recognition receptors such as the toll-like receptors (TLRs). Commensals also express TLR ligands such as LPS, PGN and flagellin, but in the context of intact barrier function, do not cause overt cellular inflammation. TLR ligands and other bacterial products clearly affect epithelial signaling, gene transcription and mediate cytoprotective effects, and TLR signaling is clearly necessary for the overall integrity of the mucosa (8, 44-46). Likely, the healthy gut requires a sub-inflammatory threshold of stimulation by bacteria or their products (TLR ligands, formylated peptide metabolic products such as butyrate) to maintain health. This concept of an optimal healthy bacterial ecosystem can be contrasted with a putative “dysbiotic” flora that can be associated with inflammatory disease (47). This is consistent with the observation that certain strains of bacteria colonized into the immature gut are associated with NEC predisposition (13, 14). In this light, Lactobacillus and other probiotics could plausibly exert beneficial effects by correcting a “dysbiotic” flora, providing predictable and beneficial exposure to bacterial factors, and consequently inducing constitutive stimulation of cytoprotective genes.
Since probiotic therapy in VLBW infants requires administration of live bacteria to patients with immature immunity, its use should not be taken lightly. While not yet reported in VLBW infants, probiotics can cause invasive disease in immunocompromised populations (48). Prebiotics (nondigestible dietary supplements which promote growth of commensal bacteria) and post-biotics (bacterial metabolites such as butyrate) can mediate beneficial effects on intestinal health. Certain LGG proteins have been shown to prevent cytokine-induced (28) and STS-induced apoptosis (data not shown) in vitro. However, our studies indicate that these bacterial products may not be as effective when administered orally to immature mice in vivo (data not shown). Future studies, which aim to better understand the beneficial actions of probiotics, without use of live microorganisms, are needed in order to develop safer alternative therapies in our patients.
Finally, the ability of LGG to induce anti-apoptotic and cytoprotective pathways without inflammation further underscores the importance of promoting growth of commensal bacteria in the developing intestine. Since apoptosis is an important precursor in the pathogenesis of NEC (5, 9, 10) and its resultant exuberant inflammatory response (septic shock, a major cause of morbidity and mortality), understanding how to inhibit apoptosis without inducing inflammation could prove invaluable in developing future preventive strategies for this devastating disease.
Acknowledgments
Financial Support: This work was supported by an Emory Egleston Children's Research Center seed grant (P.W.L.), the National Institutes of Health grants DK062851 and DK076613 (P.W.L.), AI051282 and DK071604 (A.S.N.), and Emory Digestive Disease Research Center Grant R24 DK-064399.
Abbreviations
- HPF
high power field
- LGG
Lactobacillus rhamnosus GG
- STS
Staurosporine
- VLBW
very low birthweight
REFERENCES
- 1.Lin PW, Stoll BJ. Necrotising enterocolitis. Lancet. 2006;368:1271–1283. doi: 10.1016/S0140-6736(06)69525-1. [DOI] [PubMed] [Google Scholar]
- 2.Grave GD, Nelson SA, Walker WA, Moss RL, Dvorak B, Hamilton FA, Higgins R, Raju TN. New therapies and preventive approaches for necrotizing enterocolitis: report of a research planning workshop. Pediatr Res. 2007;62:510–514. doi: 10.1203/PDR.0b013e318142580a. [DOI] [PubMed] [Google Scholar]
- 3.Neu J. Neonatal necrotizing enterocolitis: an update. Acta Paediatr Suppl. 2005;94:100–105. doi: 10.1111/j.1651-2227.2005.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 4.Dai D, Walker WA. Role of bacterial colonization in neonatal necrotizing enterocolitis and its prevention. Zhonghua Min Guo Xiao Er Ke Yi Xue Hui Za Zhi. 1998;39:357–365. [PubMed] [Google Scholar]
- 5.Jilling T, Lu J, Jackson M, Caplan MS. Intestinal epithelial apoptosis initiates gross bowel necrosis in an experimental rat model of neonatal necrotizing enterocolitis. Pediatr Res. 2004;55:622–629. doi: 10.1203/01.PDR.0000113463.70435.74. [DOI] [PubMed] [Google Scholar]
- 6.Leaphart CL, Cavallo J, Gribar SC, Cetin S, Li J, Branca MF, Dubowski TD, Sodhi CP, Hackam DJ. A Critical Role for TLR4 in the Pathogenesis of Necrotizing Enterocolitis by Modulating Intestinal Injury and Repair. J Immunol. 2007;179:4808–4820. doi: 10.4049/jimmunol.179.7.4808. [DOI] [PubMed] [Google Scholar]
- 7.Clark JA, Lane RH, Maclennan NK, Holubec H, Dvorakova K, Halpern MD, Williams CS, Payne CM, Dvorak B. Epidermal growth factor reduces intestinal apoptosis in an experimental model of necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G755–G762. doi: 10.1152/ajpgi.00172.2004. [DOI] [PubMed] [Google Scholar]
- 8.Zeng H, Wu H, Sloane V, Jones R, Yu Y, Lin P, Gewirtz AT, Neish AS. Flagellin/TLR5 responses in epithelia reveal intertwined activation of inflammatory and apoptotic pathways. Am J Physiol Gastrointest Liver Physiol. 2006;290:G96–G108. doi: 10.1152/ajpgi.00273.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ford H, Watkins S, Reblock K, Rowe M. The role of inflammatory cytokines and nitric oxide in the pathogenesis of necrotizing enterocolitis. J Pediatr Surg. 1997;32:275–282. doi: 10.1016/s0022-3468(97)90194-9. [DOI] [PubMed] [Google Scholar]
- 10.Santulli TV, Schullinger JN, Heird WC, Gongaware RD, Wigger J, Barlow B, Blanc WA, Berdon WE. Acute necrotizing enterocolitis in infancy: a review of 64 cases. Pediatrics. 1975;55:376–387. [PubMed] [Google Scholar]
- 11.Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, Fanaroff AA, Lemons JA, Donovan EF, Oh W, Stevenson DK, Ehrenkranz RA, Papile LA, Verter J, Wright LL. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129:72–80. doi: 10.1016/s0022-3476(96)70192-0. [DOI] [PubMed] [Google Scholar]
- 12.Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl. 2003;91:48–55. doi: 10.1111/j.1651-2227.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoy CM, Wood CM, Hawkey PM, Puntis JW. Duodenal microflora in very-low-birth-weight neonates and relation to necrotizing enterocolitis. J Clin Microbiol. 2000;38:4539–4547. doi: 10.1128/jcm.38.12.4539-4547.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de la Cochetiere MF, Piloquet H, des Robert C, Darmaun D, Galmiche JP, Roze JC. Early intestinal bacterial colonization and necrotizing enterocolitis in premature infants: the putative role of Clostridium. Pediatr Res. 2004;56:366–370. doi: 10.1203/01.PDR.0000134251.45878.D5. [DOI] [PubMed] [Google Scholar]
- 15.Bourlioux P, Koletzko B, Guarner F, Braesco V. Am J Clin Nutr; The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine,” held in Paris; June 14, 2002.; 2003. pp. 675–683. [DOI] [PubMed] [Google Scholar]
- 16.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science. 2001;291:881–884. doi: 10.1126/science.291.5505.881. [DOI] [PubMed] [Google Scholar]
- 17.Kelly D, Campbell JI, King TP, Grant G, Jansson EA, Coutts AG, Pettersson S, Conway S. Commensal anaerobic gut bacteria attenuate inflammation by regulating nuclear-cytoplasmic shuttling of PPAR-gamma and RelA. Nat Immunol. 2004;5:104–112. doi: 10.1038/ni1018. [DOI] [PubMed] [Google Scholar]
- 18.Collier-Hyams LS, Sloane V, Batten BC, Neish AS. Cutting edge: bacterial modulation of epithelial signaling via changes in neddylation of cullin-1. J Immunol. 2005;175:4194–4198. doi: 10.4049/jimmunol.175.7.4194. [DOI] [PubMed] [Google Scholar]
- 19.Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018–C1030. doi: 10.1152/ajpcell.00131.2005. [DOI] [PubMed] [Google Scholar]
- 20.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959–50965. doi: 10.1074/jbc.M207050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akisu M, Baka M, Yalaz M, Huseyinov A, Kultursay N. Supplementation with Saccharomyces boulardii ameliorates hypoxia/reoxygenation-induced necrotizing enterocolitis in young mice. Eur J Pediatr Surg. 2003;13:319–323. doi: 10.1055/s-2003-43580. [DOI] [PubMed] [Google Scholar]
- 22.Butel MJ, Roland N, Hibert A, Popot F, Favre A, Tessedre AC, Bensaada M, Rimbault A, Szylit O. Clostridial pathogenicity in experimental necrotising enterocolitis in gnotobiotic quails and protective role of bifidobacteria. J Med Microbiol. 1998;47:391–399. doi: 10.1099/00222615-47-5-391. [DOI] [PubMed] [Google Scholar]
- 23.Caplan MS, Miller-Catchpole R, Kaup S, Russell T, Lickerman M, Amer M, Xiao Y, Thomson R., Jr Bifidobacterial supplementation reduces the incidence of necrotizing enterocolitis in a neonatal rat model. Gastroenterology. 1999;117:577–583. doi: 10.1016/s0016-5085(99)70450-6. [DOI] [PubMed] [Google Scholar]
- 24.Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, Hammerman C. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr. 2005;147:192–196. doi: 10.1016/j.jpeds.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 25.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate. 2002;82:103–108. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 26.Hoyos AB. Reduced incidence of necrotizing enterocolitis associated with enteral administration of Lactobacillus acidophilus and Bifidobacterium infantis to neonates in an intensive care unit. Int J Infect Dis. 1999;3:197–202. doi: 10.1016/s1201-9712(99)90024-3. [DOI] [PubMed] [Google Scholar]
- 27.Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, Oh W. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2005;115:1–4. doi: 10.1542/peds.2004-1463. [DOI] [PubMed] [Google Scholar]
- 28.Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology. 2007;132:562–575. doi: 10.1053/j.gastro.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belmokhtar CA, Hillion J, Segal-Bendirdjian E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene. 2001;20:3354–3362. doi: 10.1038/sj.onc.1204436. [DOI] [PubMed] [Google Scholar]
- 30.Harkin ST, Cohen GM, Gescher A. Modulation of apoptosis in rat thymocytes by analogs of staurosporine: lack of direct association with inhibition of protein kinase C. Mol Pharmacol. 1998;54:663–670. [PubMed] [Google Scholar]
- 31.McCracken VJ, Lorenz RG. The gastrointestinal ecosystem: a precarious alliance among epithelium, immunity and microbiota. Cell Microbiol. 2001;3:1–11. doi: 10.1046/j.1462-5822.2001.00090.x. [DOI] [PubMed] [Google Scholar]
- 32.Zeng H, Carlson AQ, Guo Y, Yu Y, Collier-Hyams LS, Madara JL, Gewirtz AT, Neish AS. Flagellin is the major proinflammatory determinant of enteropathogenic Salmonella. J Immunol. 2003;171:3668–3674. doi: 10.4049/jimmunol.171.7.3668. [DOI] [PubMed] [Google Scholar]
- 33.Collier-Hyams LS, Zeng H, Sun J, Tomlinson AD, Bao ZQ, Chen H, Madara JL, Orth K, Neish AS. Cutting edge: Salmonella AvrA effector inhibits the key proinflammatory, anti-apoptotic NF-kappa B pathway. J Immunol. 2002;169:2846–2850. doi: 10.4049/jimmunol.169.6.2846. [DOI] [PubMed] [Google Scholar]
- 34.Neu J, Caicedo R. Probiotics: protecting the intestinal ecosystem? J Pediatr. 2005;147:143–146. doi: 10.1016/j.jpeds.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Boldrick JC, Alizadeh AA, Diehn M, Dudoit S, Liu CL, Belcher CE, Botstein D, Staudt LM, Brown PO, Relman DA. Stereotyped and specific gene expression programs in human innate immune responses to bacteria. Proc Natl Acad Sci USA. 2002;99:972–977. doi: 10.1073/pnas.231625398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Q, Liu D, Majewski P, Schulte LC, Korn JM, Young RA, Lander ES, Hacohen N. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 37.Eckmann L, Smith JR, Housley MP, Dwinell MB, Kagnoff MF. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J Biol Chem. 2000;275:14084–14094. doi: 10.1074/jbc.275.19.14084. [DOI] [PubMed] [Google Scholar]
- 38.Mazumder S, Plesca D, Kinter M, Almasan A. Interaction of a cyclin E fragment with Ku70 regulates Bax-mediated apoptosis. Mol Cell Biol. 2007;27:3511–3520. doi: 10.1128/MCB.01448-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pramoonjago P, Baras AS, Moskaluk CA. Knockdown of Sox4 expression by RNAi induces apoptosis in ACC3 cells. Oncogene. 2006;25:5626–5639. doi: 10.1038/sj.onc.1209566. [DOI] [PubMed] [Google Scholar]
- 40.Podcheko A, Northcott P, Bikopoulos G, Lee A, Bommareddi SR, Kushner JA, Farhang-Fallah J, Rozakis-Adcock M. Identification of a WD40 repeat-containing isoform of PHIP as a novel regulator of beta-cell growth and survival. Mol Cell Biol. 2007;27:6484–6496. doi: 10.1128/MCB.02409-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tewari M, Yu M, Ross B, Dean C, Giordano A, Rubin R. AAC-11, a novel cDNA that inhibits apoptosis after growth factor withdrawal. Cancer Res. 1997;57:4063–4069. [PubMed] [Google Scholar]
- 42.Ruchatz H, Coluccia AM, Stano P, Marchesi E, Gambacorti-Passerini C. Constitutive activation of Jak2 contributes to proliferation and resistance to apoptosis in NPM/ALK-transformed cells. Exp Hematol. 2003;31:309–315. doi: 10.1016/s0301-472x(03)00007-9. [DOI] [PubMed] [Google Scholar]
- 43.Cerignoli F, Rahmouni S, Ronai Z, Mustelin T. Regulation of MAP kinases by the VHR dual-specific phosphatase: implications for cell growth and differentiation. Cell Cycle. 2006;5:2210–2215. doi: 10.4161/cc.5.19.3267. [DOI] [PubMed] [Google Scholar]
- 44.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 45.Abreu MT, Vora P, Faure E, Thomas LS, Arnold ET, Arditi M. Decreased expression of Toll-like receptor-4 and MD-2 correlates with intestinal epithelial cell protection against dysregulated proinflammatory gene expression in response to bacterial lipopolysaccharide. J Immunol. 2001;167:1609–1616. doi: 10.4049/jimmunol.167.3.1609. [DOI] [PubMed] [Google Scholar]
- 46.Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, Akira S, Takeda K, Lee J, Takabayashi K, Raz E. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126:520–528. doi: 10.1053/j.gastro.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 47.Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 48.Kliegman RM, Willoughby RE. Prevention of necrotizing enterocolitis with probiotics. Pediatrics. 2005;115:171–172. doi: 10.1542/peds.2004-2271. [DOI] [PubMed] [Google Scholar]