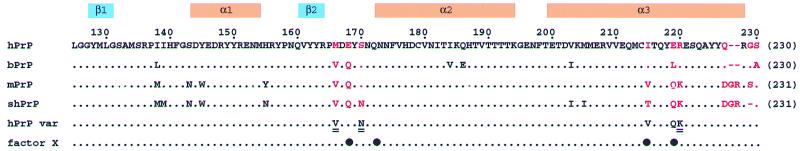
Figure 1.
Amino acid sequence alignment of the fragment 125–230 (numeration of hPrP following ref. 8; for each protein the number in parentheses on the right indicates the sequence position attributed to the C-terminal residue) of the human, bovine, murine, and Syrian hamster PrPs. At the top the locations of the regular secondary structure elements of hPrP(121–230) are indicated. The red letters identify residue positions with amino acid exchanges or insertions in the region of the three-dimensional structure that are discussed in this paper. The row “hPrP var” lists the amino acid exchanges in the single-residue variants of hPrP studied in this paper, where the doubly underlined residues indicate those variants for which a complete structure determination is presented. In the row “factor X” the black circles identify the sequence positions 168, 172, 215, and 219, which have been suggested to form an epitope or part of an epitope for interactions with a species-specific “factor X” or “protein X” (15, 33).