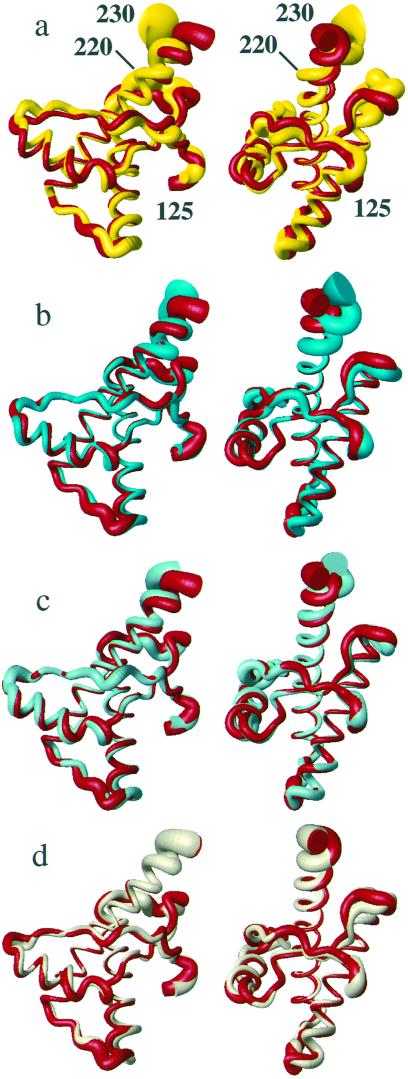
Figure 3.
Superposition of the polypeptide backbone from residues 125–230 in different PrP NMR structures for best fit of the backbone heavy atoms of residues 125–220. The radius of the cylindrical rods representing the polypeptide chains is proportional to the mean global backbone displacement per residue (35) among the 20 energy-minimized conformers used to represent the NMR structures. The views on the right were obtained from those on the left by a −90° rotation about a vertical axis. (a) hPrP(121–230) (red) and mPrP(121–231) (yellow). (b) hPrP(121–230) (red) and hPrP(R220K) (cyan). (c) hPrP(121–230) (red) and hPrP(M166V) (turquoise). (d) hPrP(121–230) (red) and hPrP(S170N) (amber). For these comparisons we recalculated the NMR structures of mPrP(121–231) (2) and hPrP(121–230) (6), using the same protocol as for the hPrP(121–230) variants (see Materials and Methods).