Abstract
Glioblastoma multiforme (GBM) is the most common form of primary brain cancer. In the past decade, virotherapy of tumors has gained credence, particularly in glioma management, as these tumors are not completely resectable and tend to micro-metastasize. Adenoviral vectors have an advantage over other viral vectors in that they are relatively non-toxic and do not integrate in the genome. However, the lack of coxsackie and adenovirus receptors (CAR) on surface of gliomas provides for inefficient transduction of wild-type adenoviral vectors in these tumors. By targeting receptors that are over-expressed in gliomas, modified adenoviral constructs have been shown to efficiently infect glioma cells. In addition, by taking advantage of tumor specific promoter (TSP) elements, oncolytic adenoviral vectors offer the promise of selective tumor-specific replication. This dual targeting strategy has enabled specificity in both laboratory and pre-clinical settings. This review looks at current trends in adenoviral virotherapy of gliomas, with an emphasis on targeting modalities and future clinical applications.
Malignant glioma
Malignant gliomas are one of the most aggressive cancers for which limited therapeutic improvement has been made over the past decade. Glioblastoma multiforme (GBM, astrocytoma grade IV) accounts for the majority of all primary brain tumors. Although new drugs like bis-chloronitrosourea (BCNU, Gliadel) and temozolomide (Temodar) have been approved by the Food and Drug Administration (FDA) in recent years, they provide only marginal benefit, with the majority of patients eventually dying from the disease.
Treatment of gliomas
Conventional treatment of GBMs includes surgical resection and radiation therapy. However, due to its location and its tendency to infiltrate the surrounding brain tissue, complete surgical removal is frequently not possible. Even after surgical debulking of the tumor, GBMs tend to micro-metastasize within the brain [1]. Standard radiation therapy is usually in the form of high dose radiation ranging from 50 Gy to 60 Gy [2] and is usually concurrent with chemotherapy. However, radiation is not a complete therapy as a fraction of glioma cells remain radioresistant and evade cellular damage and eventually re-form the tumor. This radioresistance could be due to the presence of CD133+ brain tumor stem cells [3] or other mutations like p53 [4] or the upregulation of the tumor specific protein survivin [5], the complete discussion of which is beyond the scope of this review.
The current course of chemotherapy in gliomas consists of temozolomide (TMZ) according to the Stupp protocol [6]. Compared to radiotherapy alone, combination of TMZ and radiation has prolonged median survival to 14 months [7]. Other drugs that are currently FDA approved and in use include Gliadel [8,9]. Gliadel, in combination with radiation and TMZ, has also been used in phase I/II clinical trials and this combination has improved median survival to 21.4 months compared with 12.4 months with radiation and Gliadel alone [7].
Virus as a therapeutic tool
The advent of recombinant DNA technology has enabled pathogenic virus to be harnessed for human good. It has been possible for some time now to create viruses that retain infectivity while attenuating their pathogenicity. This strategy has been utilized to target tumors and express a gene of interest inside the tumor. Alternatively, viruses that are conditionally replicative (CRAds) have been used to multiply inside tumor cells and eventually kill these cells, a method termed virotherapy. In this section, we summarize the different strategies and the different types of viruses used to date.
Treatment of brain tumors using virotherapy has a long history. The earliest publication of virus-glioma interaction was in 1961 when rabies virus was studied in glial tumors [10]. Subsequently, growth of herpes simplex virus and measles virus was also examined in gliomas and other cells of neural origin although the oncolytic effects were not clearly established [11-15]. Although these studies were intended to study growth of viruses more than advance the cause of glioma therapy, they provided valuable resources for subsequent research that looked into virotherapy of gliomas. The first research exclusively focusing on virotherapy of glioma was conducted in Japan in 1982 using mumps virus, a member of the paramyxoviridae family of viruses [16].
The most commonly studied virus in glioma virotherapy has been the herpes simplex virus (HSV). The first of these studies involved a mutation in the thymidine kinase (tk) gene, dlsptk that resulted in attenuated neurovirulence. This vector killed glioma cells in vitro and controlled tumor growth in vivo in an intracranial model of glioma [17]. Further studies showed that mutation in the herpes viral gene γ134.5 reduced neurotoxicity and its capacity to inhibit tumor growth but most importantly retained sensitivity to common anti-virals used for herpes infections [18]. Most studies with oncolytic HSV have been carried out using this mutation as a backbone and have been elegantly reviewed by Markert [19]. Other major studies on virotherapy of gliomas have been conducted using retrovirus [20-22] and have been reviewed in Rainov and Ren [23]. Measles virus [24], reovirus [25,26], adeno-associated virus [27], Newcastle disease virus (NDV) [28,29], Semliki Forest virus [30], vaccinia virus [31-33] and poliovirus [34,35] have also been used. However, the discussion of these viruses as an effective oncolytic strategy for gliomas is beyond the scope of this review.
Adenovirus as a therapeutic tool
Recombinant adenovirus has been explored as an alternative to conventional chemotherapy and radiotherapy and their combination for almost a decade. Adenovirus has a double stranded DNA genome of 36kbp. Adenovirus serotype-5 (Ad5), the most common of the various subtypes, belongs to subgroup C and infects human cells using the coxsackie and adenovirus receptor (CAR), a 46kDa cell-surface receptor [36-38]. This binding, along with a secondary interaction between the virus penton base proteins and host surface integrins (αvβ3 and αvβ5), is responsible for adenoviral internalization into cells [39]. The entry of an adenovirus into tumor cells is, however, complicated by the reduction or total absence of CAR in tumor cells in general and GBMs in particular [40]. Targeting the adenovirus to human tumors therefore remains a challenge.
An elegant yet simplistic approach taken to overcome resistance to infection by Ad5 to refractory cells, like tumor cells, is the modification of the Ad5 knob. Since the CAR levels in gliomas are low, the knob is a redundant element of the Ad5 viral structure for the purpose of cancer therapy and therefore can be modified or replaced without loss of infectivity. Strategies to enhance Ad infection in tumor cells involve modification of this CAR binding domain in the knob region of Ad5. A wide variety of approaches have been taken so far to modify Ad5 knob. One of these approaches involves incorporating the Arg-Gly-Asp (RGD) motif into the Ad knob in order to bind αvβ3 and αvβ5 integrins expressed on tumor cells [41]. By modifying the HI loop of Ad5 to incorporate this RGD motif, it is possible to markedly improve Ad tropism to tumor cells in a CAR-independent mechanism. The CRAd, AdΔ24 was also enhanced by the addition of the RGD moiety and tested in panel of glioma cell lines and in an in vivo xenograft model [42]. In 10 primary glioma samples tested, cellular toxicity increased by at least 10% to a maximum of 70% in AdΔ24 RGD compared to AdΔ24 alone. In vivo, the tumor burden decreased dramatically over a period of 120 days compared to no virus injection as control [42].
Another modification of the knob that enhances tropism of Ad5 is the poly-lysine modification (pK7), whereby addition of multiple lysine residues enhances the electrostatic attraction between the knob and the anionic cell surface receptors like heparan sulfate proteoglycans [39, 43]. A vector which incorporates the pK7 modification (AdZ.FpK7) infected U87MG and GL261 cell lines at significantly higher levels than AdZ.F control in vitro and in an in vivo subcutaneous model [44]. A hybrid fiber-knob domain comprising of Ad5 fiber shaft and Ad3 knob has been used to enhance binding to gliomas. The Ad3 knob has an affinity for binding to CD46, CD80 and CD86 receptors, resulting in more efficient binding and replication compared to that of the wild-type Ad5 virus. First tested in head and neck cancers, Ad5/3 displayed higher infectivity by several hundred-fold over Ad5 vectors [45]. Ad5/3 was also tested in a panel of cell lines including, the U118MG glioma line, and showed an increase in viral tropism vs. Ad5[46]. The authors also generated a mosaic virus by recombining Ad5 and Ad 5/3. These adenoviruses had different fractions of Ad5 and Ad3 fiber on the same viral particle that resulted in significantly higher infectivity in a panel of cell lines, including U118MG. The Ad5/3 chimera was also tested in a panel of glioma cell lines and primary GBM samples for infectivity [47]. The cell lines tested all showed high CD46 levels, making them a good target for this vector. Later studies also showed that CD80/86 levels were high in these cells, an often discussed potential target for the Ad5/3 chimeras [48]. Although in vitro results were not significantly higher than Ad5 alone, ex vivo analysis of GBM samples showed a 10-fold increase in infectivity compared to the Ad5 vector. Comparison of Ad pK7, AdRGD, and Ad5/3 revealed that while different modifications to the knob work better in some cell lines, there is an increase in viral infectivity in all the modified vectors compared to the Ad5 knob alone [47].
Using the σ1 protein, the receptor-binding molecule of serotype 3 bearing reovirus, in fusion with Ad5 fiber, a recombinant Ad was created that binds to sialic acid and JAM1 molecules in human cell lines including glioma [49]. This fusion Ad 5-Sigma vector was also found to have increased infectivity in a panel of glioma cells compared to wild type Ad5 [47].
Another approach to increase the tropism of adenovirus to glioma cells is the use of “xenotype” knobs. In this method, the knob from a different species of adenovirus is linked to the wild-type human Ad5 fiber. Using the canine, porcine, murine and ovine adenovirus knobs has enabled greater transduction efficiency of these modified viruses [50, 51]. The canine adenovirus type I knob, CAV1, was fused to Ad5 to generate Ad5-CAV1 and tested by Zheng et al., [47] for increased tropism to good effect. The canine and porcine knobs were both found to enhance infectivity along with Ad5/3 compared to Ad5 alone in four glioma cell lines tested as well as in primary GBM tissues [50].
An undesired advantage in cancer cells is their upregulation of several receptors that play a role in aberrant growth and migration. This is also true for gliomas and several such receptors, like EGFR, PDGFR, FGFR, IGFR and VEGFR have been found to be highly expressed in primary gliomas [52]. Modifying Ads that target these receptors bypasses CAR dependency in adenoviral transduction. One of these approaches uses the EGFR receptor that was found to be over-expressed in gliomas [53]. In CAR deficient but EGFR expressing cell lines, the potency of this adenoviral vector is about 1000-fold higher than the control vector that has no EGFR biding domain in the fiber. Fibroblast growth factor (FGF) is another such receptor and has been used to modify the Ad knob for better targeting [54]. The FGF receptor 1 (FGFR1) was shown to be over-expressed in four glioma cell lines as well as in 7 GBM samples tested. By chemically conjugating the FGF2-Fab’ antibody to wild-type Ad expressing GFP, the modified virus FGF2-AdGFP was created. The number of GFP positive cells using this modified vector was higher than AdGFP infected cells by at least 5% (U87MG) to as high as 80% in a primary GBM culture. In an intracranial model of mouse glioma, using U118MG cells, the number of GFP positive cells increased dramatically using the recombinant vector than the wild-type GFP expressing vector or PBS controls [54]. More recently, a mutant variation of EGFR, EGFRvIII, has been found to be upregulated in up to 50% of gliomas. This presents a new and attractive target for knob modifications [55, 56]. Although these approaches bypass the need for CAR and have met with varying successes both in vitro and in vivo, tumor biologists have not stopped looking for more innovative solutions.
Successful entry of Ad5 or its derivatives is just one of many steps for Ad virotherapy to be an effective tool in glioma therapy. A second and equally important step is its replication once inside the cells. Efficient replication with subsequent viral progeny release is essential to cell killing and effective glioma therapy. Consequently, a number of innovative approaches have been taken in this regard. Initially, replication incompetent viruses were used for glioma therapy. These viruses were genetically modified with a mutation in the early promoter E1A or both E1A and E1B [57, 58]. A replication incompetent adenovirus for example, carrying the cytochrome P450 gene into glioma cells, increased the response of these cells to cyclophosphamide [59]. Several other approaches were used for replication incompetent adenovirus [60-65]. Most notable among them was the approach taken by Zhang et al. [66] in which the entire E1 region of Ad5 was deleted and replaced by the p53 gene driven by the cytomegalovirus (CMV) promoter. This construct, Ad-p53 (also known as Advexin), was tested in a variety of cancers including gliomas. In vitro,Ad-p53 induced apoptosis in glioma cells and inhibited the growth of glioma xenografts in vivo [67, 68]. A phase I clinical trial of 15 patients was conducted showing minimal toxicity and a median recurrence time of 7 months with one patient tumor free after 30 months. The biggest drawback of the study was the lack of diffusion of the viral bolus a few millimeters beyond the injection site [68, 69].
In order to overcome the limitations of spread, several groups have focused on the importance of connective tissue and extracellular matrix (ECM). For example, a study on the role of ECM in inhibiting viral spread was done by Kuriyama[70]. The authors observed that addition of proteases such as trypsin and a mixture of collagenase-dispase aided the viral diffusion process. In a separate study, an ECM degrading enzyme, relaxin was used in a replication incompetent (dl-lacZ-RLX) and competent (Ad-ΔE1B-RLX) virus. Increased transduction and viral spread throughout the tumor mass was observed in these subcutaneous models of glioma, showing the importance of the ECM in preventing viral spread [71]. Increased expression of MMP-1 and MMP-8 has also been correlated with increased viral distribution by modulating the ECM [72].
Trask [73] conducted a phase I study in which a replication deficient Ad bearing the HSV-tk gene driven by the Rous Sarcoma Virus (RSV) promoter (Adv.RSVtk) was injected intratumorally followed by ganciclovir (GCV) treatment. The virus was safely tolerated even at the highest dose but failed to achieve significant tumor cell death. A similar study with HSV-tk gene, this time with the CMV promoter, was conducted by Sandmair [74]. There was an increase in frequency of epileptic attacks and a ‘tendency’ for improved survival was reported [74]. Another trial was conducted by controlling the HSV-tk gene using the Ad major late promoter (MLP) [64]. The study concluded that patients tolerated the virus and the subsequent GCV treatment, but did not proceed further. Similar studies by Germano et al.,[75] and Immonen et al.,[76] using the Ad vector used by Sandmair, showed little to moderate improvement in survival rates and moderate tolerability. A replication competent version of this Ad was tried in lung cancer and melanoma [77] and in gliomas [78]. In the latter case, it showed increased cytotoxicity, reduced glioma volume, and increased survival in a mouse xenograft model.
These trials with non-replicating adenoviruses failed to significantly change prognosis and the problem of glioma recurrence due to its disseminating nature was not addressed. This underscored the need for replication competent viruses that would infect cells, lyse them, and release viral progeny that can further infect cells in the vicinity and thereby cause the virus to spread. This replicative cycle can potentially prevent recurrences due to micrometastatic lesions. Another huge disadvantage with these viruses was that while they were able to target normal brain and low-grade tumors, they were unable to infect highly aggressive gliomas [58, 79]. In light of these limitations, CRAds have entered the clinical scene.
Conditionally replicative adenoviruses (CRAds)
CRAds replicate under certain conditions that are usually found in cancer cells and absent in normal healthy cells. These viruses may have either (1) inessential regions of their genomes deleted, or (2) transduce only cancer cells and spare normal cells because of the over-expression of receptors on the cell surface of gliomas, or (3) tumor specific promoter driving an essential gene in the Ad genome (for example E1A early promoter) [42, 80-88]. Earlier in this review we have discussed the second of the three points mentioned here and therefore the remainder of this review will be dedicated to remaining points.
The human adenovirus E1B 55kDa protein interacts with the tumor suppressor p53 and blocks its transcriptional activity. Deletion of this region in the Ad5 genome prevents this interaction and this principle has been used to create a CRAd that replicates in glioma cells that lack p53 protein. Deletion of this 55kDa protein in Ad5 led to the creation of the adenovirus dl1520, also known as ONYX-015. This virus replicated efficiently in gliomas that are attenuated for p53 but not in p53 wild-type tumors or normal human cells that have normal levels of p53 [89]. Initial studies with ONYX-015 showed significant tumor cell killing and reduction in tumor mass in preclinical experiments both in vivo and in vitro [90]. In clinical studies, ONYX-015 has been used in a phase I clinical trial [91]. Safety and efficacy being the objective of this study, they were met satisfactorily in all 24 patients enrolled, 17 of which were grade IV glioma (GBM) patients. The virus was well tolerated even at its highest dose of 1010 pfu. Ten out of 24 patients had some adverse effects but were eventually determined to be not related to the virus treatment. However, only about 50% gliomas are p53 negative, making this virus ineffective in the remaining 50% that are p53 positive, reducing the scope of its application.
Another more successful conditionally replicative approach was undertaken by Fueyo and collaborators. In this approach, a 24-bp deletion in the Ad 5 E1A CR2 domain inhibited the binding of E1A protein to the retinoblastoma protein (pRb). Since the pRb protein negatively regulates cell growth by releasing E2F, a failure to release this inhibition stops cell growth [81]. This E1A mutant Ad, known as Ad5-Δ24 replicates only in tumor cells and not in normal brain cells [81]. Both in vitro and in vivo studies with this tumor have shown potent cytolytic activities, particularly in vivo where a single low dose local injection inhibited tumor grown in a mouse graft model of glioma [81]. Building on the success of these preclinical studies, a modified virus Ad5-Δ24RGD was created that has dual transductional targeting. Addition of this modification increased the cytotoxic effect of the virus in glioma cell lines and in vivo experiments showed 9 out of 10 animals showing complete tumor regression [42]. A still further modification of this virus was the ICOVIR series: ICOVIR-2 and ICOVIR-5. In addition to the modifications in Ad5-Δ24RGD, ICOVIR-5 has an E2F responsive element in lieu of the E1A promoter that further confines the replication of this Ad in tumor cells [92]. In vitro, ICOVIR-5 showed an increased anti-glioma effect and increased replication in glioma cell lines; however in vivo, there was no significant increase over the second generation Ad5-Δ24RGD virus in controlling tumor growth and long term survival was lower for ICOVIR-5 than Ad5-Δ24RGD (46.5d vs. 71.0d) in a mouse xenograft model of glioma [92]. A summary of adenoviral vectors in clinical trials is listed in Table 1.
Table 1.
Adenovirus in clinical trials for glioma.
| Gene/Prodrug | Clinical Trial | Reference |
|---|---|---|
| Replication Defective Adenovirus | ||
| Adv.RSVtk/Gancyclovir | Phase I, dose escalation | Trask et al, 2000 [73]. |
| Ad-p53 (INGN 201; Advexin) | Phase I, dose escalation | Lang et al, 2003 [69]. |
| IG.Ad.MLPI.TK/Gancyclovir | Phase I, dose escalation | Smitt et al, 2003 [64]. |
| ADV/HSV-tk/Gancyclovir | Phase I, dose escalation | Germano et al, 2003 [75]. |
| HSV-tk/Gancyclovir (Cerepro) | Phase III (European Medicines Agency) | Immonen et al, 2004 [76]. |
| AdV-tk/Valacyclovir (GliAtak) | Phase I, dose escalation Phase Ib, currently ongoing |
New et al, 2008 [93]. Protocol ID: NCT00751270 |
| AdV-tk /Valacyclovir/Radiation (GliAtak) | Phase II, currently ongoing | Protocol ID: NCT00589875 |
| Replication Competent Adenovirus | ||
| Delta-24-RGD | Phase I, currently ongoing | Protocol ID: NCT00805376 |
| ONYX-015 | Phase I, open label, dose escalation | Chiocca et al, 2004 [91]. |
Brain tumor specific promoters (TSP), like nestin and GFAP, have been previously used in non-replicating adenoviruses to test the hypothesis of tumor specificity and glioma targeting [94]. These TSPs can be used to drive the E1A gene of Ad5, which would then replicate selectively in glioma cells only where the levels of these proteins are high and not in normal brain tissues. The midkine promoter is one such protein over-expressed in malignant gliomas and a CRAd driven by this promoter showed strong virolytic effects in glioma cells but not in midkine negative normal brain cells. In animal experiments, Ad-MK ablated tumor xenografts in midkine expressing cells [85]. The loss of pRb in tumor cells potentially leads to an excess of free E2F in tumor cells. This hypothesis was elegantly tested by Parr et al., [86] in a rat glioma model. Using a βgal reporter system, an Ad.E2F1.βgal adenoviral vector specifically targeted tumor cells in an in vivo model while sparing normal brain tissues when compared to a similar construct driven by the CMV promoter or wild type Ad5. The human telomerase RNA (hTR) and the human telomerase reverse transcriptase (hTERT) is active in a vast majority of cancer cells including gliomas, however, their use in CRAds for glioma specific treatment has been proposed but not yet achieved [95, 96].
Survivin is a tumor specific promoter that has recently come into prominence [5, 97-99]. It has been used in in vitro and in vivo studies in glioma models and has shown promise in targeting gliomas [100-102]. Specifically, a CRAd with its E1A driven by the survivin promoter and its knob modified with a pK7 moiety (CRAd-S-pK7) has been shown to have increased targeting to glioma cell lines and to improve survival of animals with intracranial tumors [102, 103].
A very ingenious system was developed by Wohlfahrt et al., [104] in which an Ad5 vector was deleted for all E1A and E1B genes. By use of inverted repeats, this virus replicated only in tumor cells and produced E1A, which in turn synergistically allowed more viral replication. By placing tumor necrosis factor related apoptosis-inducing ligand (TRAIL) under the control of this E1A promoter, the replicating cells apoptosed and released TRAIL to the surrounding cells [104]. This virus also used the chimeric Ad 5/Ad 35 knob: a dual modification that promotes more specificity. Such a therapeutic approach was also used along with an Ad5 vector expressing Bax and Caspase-8. Shinoura et al. [87] showed increased cell killing by apoptosis in glioma cell lines using this virus.
Delivery systems using CRAds are an exciting approach and therapeutic genes such as p53 and TRAIL have been tested in gliomas [105]. More recently, a combination of CRAd and TMZ, the drug of choice in gliomas, was tested both in vitro and in vivo in a mouse xenograft model [106]. The authors observed an increase in toxicity in the combination treatment group as compared to CRAD-S-pK7 or TMZ treatment alone. There was also an increase in long-term survival in a mouse model of glioma after treatment with the combination therapy. This approach should be further tested in a pre-clinical setting.
Limitations of virotherapy
Despite the large body of research cited here on adenoviral therapy of malignant gliomas, the challenge remains unabated. All GBM tissues do not uniformly over-express the same receptors; therefore they cannot be transduced uniformly by a single fiber modification. Similarly, even after viral entry, efficient adenoviral replication remains a challenge. All glioma cells do not express the same TSPs, nor are they uniformly high. Furthermore, because GBMs tend to disseminate into the brain parenchyma, tumor cells remain out of reach and newer delivery methods have to be devised to overcome this problem [107, 108].
In order to overcome the limitations associated with deliver of oncolytic vectors, mesenchymal stem cells (MSC) were used as a carrier of CRAds. These cells have an inherent tropism towards gliomas using growth factors as chemo-attractants. Since MSCs can be easily isolated in an autologous manner and they possess the potential to migrate to tumor-bearing regions of the brain inaccessible by surgical intervention, they are an attractive candidate as a delivery vehicle. MSCs were therefore tested in a mouse model of intracranial glioma [107]. At a viral concentration of 100vp/MSC, a significant increase in viral delivery to distal regions of the brain was achieved compared to a similar concentration of virus alone. This concentration of virus was also found optimal as it reduced cytotoxicity to the host MSCs long enough for them to be able to migrate to these distally located glioma cells in the mouse brain.
The second strategy involves using neural stem cells (NSC) instead of MSCs. These cells have also been shown to have a preference for migration towards gliomas in vivo in response to the cytokines released by the tumor [109]. In the study by Tyler et al. [108], NSCs were shown to be able to efficiently migrate towards gliomas and target them by releasing their viral load via a suicide mechanism common for all adenoviral infections. This study also showed that CRAd-loaded NSCs target tumors that are located away from the site of injection of the CRAd-bearing NSCs. Therefore, both of these approaches could be extremely beneficial in targeting and killing glioma cells that are embedded in the brain away from surgical and drug injection sites and need to be evaluated in greater detail.
Attempts to modulate the immune system in order to both augment the anti-tumor immune response while at the same time decrease the anti-viral immune response are an important and ongoing area of research. Using an adenovirus expressing IL-12, GBM cells have been shown to elicit an anti-tumor immune response [110]. Adenoviral expression of the human decorin gene, a known TGF-β antagonist, has also been found to induce an anti-tumor immune response [111]. Pretreatment of gliomas using cyclophosphamide (CPA) resulted in increase in infection of tumor cells by HSV in both athymic and syngeneic models of brain tumor. This increase was found due to immunosupression and down-regulation of IFNs α, β, γ, TNFα, IL-15 and IL-18 genes by CPA [112]. In an adenovirus model, injection of CPA decreased the rate of viral depletion as evidenced by luciferase expression. This effect was found in both replication-deficient and replication-competent adenoviruses AdΔ24CMV-Luc and Ad.CMV-Luc, respectively in athymic mouse model [113]. Another immunotherapy approach consists in the injection of an adenovirus expressing IFN-β to the tumor bed. In a phase I study, there was an increase in apoptotic cells with an associated increase in tumor necrosis [114]. However, detailed studies on the anti-tumor immune response mechanism in glioma are still lacking.
Previous studies have demonstrated that MSCs inhibit mitogen-and alloantigen-induced T cell proliferation [115-118] and NK cell proliferation [119]. Our laboratory has sought to exploit the immunosuppressive capabilities of MSC to enhance the persistence of oncolytic adenoviral vectors in vivo. Using the permissive cotton rat model, we have confirmed that MSC loaded with oncolytic adenoviral vectors retain the ability to suppress T cell activation, proliferation, and IFN-γ production in response to mitogenic stimulation in vitro (unpublished observation). Studies are currently underway to assess the immunosuppressive ability of MSC in vivo.
The future of adenovirus therapy for gliomas (Expert Opinion)
The history of adenovirus as a therapy for gliomas is a short one; however it is one that holds promise. Clinical trials with ONYX-015 have established that adenoviruses are safe for use in humans and that they have minimal toxic side-effects [91]. The key to success in virotherapy in gliomas is delivery and specific targeting of tumor cells. The fact that after surgical resection these tumors have a tendency to micrometastasize and disseminate into healthy tissues in the brain makes it especially so. However, a huge ‘advantage’ in targeting gliomas is that the surrounding healthy brain tissue is quiescent as opposed to the actively replicating tumor cells. Replicative adenovirus therapy is therefore the key to this strategy. As mentioned earlier, due to the non-uniform expression of cell surface receptors and tumor specific promoters, a single CRAd treatment is unattainable. All options are to be analyzed for a particular patient with GBM and individualized molecular-profiled treatment is therefore the correct approach. Although a lot of progress has been made in the laboratory settings, clinical trials with CRAds need to go forward. Researchers also cannot be complacent with their successes in non-clinical setting, as clinical prognosis has remained grim. Alternate transductional and transcriptional targets needs to be identified. Another aspect that needs special attention is the delivery of these viruses so that they reach the lesion targeted. Recently, stem cells have become an attractive delivery vehicle and this line of research needs to be pursued. Adenovirotherapy for gliomas holds promise in the near future and should be actively pursued on all fronts.
Figure 1.
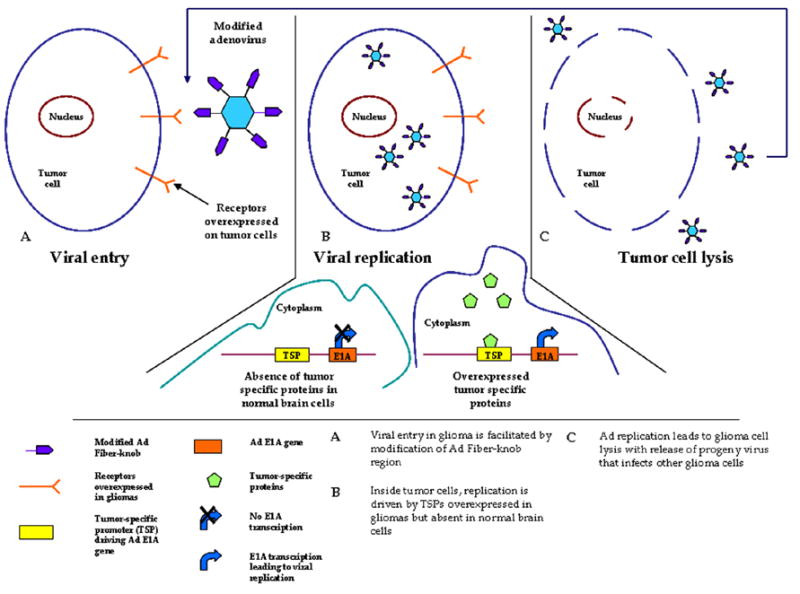
Schematic overview of CRAds in glioma.
Acknowledgments
This work was supported by the National Cancer Institute (R01-CA122930), the National Institute of Neurological Disorders and Stroke (K08-NS046430), The Alliance for Cancer Gene Therapy Young Investigator Award, and the American Cancer Society (RSG-07-276-01-MGO).
References
- 1.Thorsen F, Tysnes BB. Brain tumor cell invasion, anatomical and biological considerations. Anticancer Res. 1997 Nov-Dec;17(6B):4121–6. [PubMed] [Google Scholar]
- 2.Chang JE, Khuntia D, Robins HI, Mehta MP. Radiotherapy and radiosensitizers in the treatment of glioblastoma multiforme. Clin Adv Hematol Oncol. 2007 Nov;5(11):894–902. 7–15. [PubMed] [Google Scholar]
- 3.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008 Jun 10;26(17):2839–45. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hulleman E, Helin K. Molecular mechanisms in gliomagenesis. Adv Cancer Res. 2005;94:1–27. doi: 10.1016/S0065-230X(05)94001-3. [DOI] [PubMed] [Google Scholar]
- 5.Das A, Tan WL, Teo J, Smith DR. Expression of survivin in primary glioblastomas. J Cancer Res Clin Oncol. 2002 Jun;128(6):302–6. doi: 10.1007/s00432-002-0343-4. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005 Mar 10;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 7.McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2008 Dec 1; doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003 Apr;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brem H, Piantadosi S, Burger PC, Walker M, Selker R, Vick NA, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995 Apr 22;345(8956):1008–12. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 10.Aksel IS, Aykan TB. Growth behavior of the rabies virus in a glioblastomatous tumor induced with methylcholanthrene in mice. World Neurol. 1961 May;2:398–405. [PubMed] [Google Scholar]
- 11.Mannweiler K, Palacios O. Cultivation and reproduction of herpes simplex virus in nervous system cell cultures. Acta Neuropathol. 1969;12(3):276–99. doi: 10.1007/BF00687650. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Homma M, Ishida N. Growth of measles virus in cultures of rat glioma cells. Infect Immun. 1975 Sep;12(3):614–20. doi: 10.1128/iai.12.3.614-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleury H, Pasquier PD. Replication of measles virus in a cell culture from a glioblastoma of human origin. J Neuropathol Exp Neurol. 1977 Sep-Oct;36(5):842–5. doi: 10.1097/00005072-197709000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Adler R, Glorioso JC, Levine M. Infection by herpes simplex virus and cells of nervous system origin: characterization of a non-permissive interaction. J Gen Virol. 1978 Apr;39(1):9–20. doi: 10.1099/0022-1317-39-1-9. [DOI] [PubMed] [Google Scholar]
- 15.Tsiang H, Koulakoff A, Bizzini B, Berwald-Netter Y. Neurotropism of rabies virus. An in vitro study. J Neuropathol Exp Neurol. 1983 Jul;42(4):439–52. doi: 10.1097/00005072-198307000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Yumitori K, Handa H, Yamashita J, Suda K, Otsuka S, Shimizu Y. Treatment of malignant glioma with mumps virus (author’s transl) No Shinkei Geka. 1982 Feb 10;10(2):143–7. [PubMed] [Google Scholar]
- 17.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991 May 10;252(5007):854–6. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 18.Markert JM, Malick A, Coen DM, Martuza RL. Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993 Apr;32(4):597–603. doi: 10.1227/00006123-199304000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Markert JM, Parker JN, Buchsbaum DJ, Grizzle WE, Gillespie GY, Whitley RJ. Oncolytic HSV-1 for the treatment of brain tumours. Herpes. 2006 Nov;13(3):66–71. [PubMed] [Google Scholar]
- 20.Oldfield EH, Ram Z, Culver KW, Blaese RM, DeVroom HL, Anderson WF. Gene therapy for the treatment of brain tumors using intra-tumoral transduction with the thymidine kinase gene and intravenous ganciclovir. Hum Gene Ther. 1993 Feb;4(1):39–69. doi: 10.1089/hum.1993.4.1-39. [DOI] [PubMed] [Google Scholar]
- 21.Raffel C, Culver K, Kohn D, Nelson M, Siegel S, Gillis F, et al. Gene therapy for the treatment of recurrent pediatric malignant astrocytomas with in vivo tumor transduction with the herpes simplex thymidine kinase gene/ganciclovir system. Hum Gene Ther. 1994 Jul;5(7):863–90. doi: 10.1089/hum.1994.5.7-863. [DOI] [PubMed] [Google Scholar]
- 22.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000 Nov 20;11(17):2389–401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 23.Rainov NG, Ren H. Clinical trials with retrovirus mediated gene therapy--what have we learned? J Neurooncol. 2003 Dec;65(3):227–36. doi: 10.1023/b:neon.0000003652.71665.f2. [DOI] [PubMed] [Google Scholar]
- 24.Phuong LK, Allen C, Peng KW, Giannini C, Greiner S, TenEyck CJ, et al. Use of a vaccine strain of measles virus genetically engineered to produce carcinoembryonic antigen as a novel therapeutic agent against glioblastoma multiforme. Cancer Res. 2003 May 15;63(10):2462–9. [PubMed] [Google Scholar]
- 25.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998 Nov 13;282(5392):1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 26.Yang WQ, Lun X, Palmer CA, Wilcox ME, Muzik H, Shi ZQ, et al. Efficacy and safety evaluation of human reovirus type 3 in immunocompetent animals: racine and nonhuman primates. Clin Cancer Res. 2004 Dec 15;10(24):8561–76. doi: 10.1158/1078-0432.CCR-04-0940. [DOI] [PubMed] [Google Scholar]
- 27.Mizuno M, Yoshida J, Colosi P, Kurtzman G. Adeno-associated virus vector containing the herpes simplex virus thymidine kinase gene causes complete regression of intracerebrally implanted human gliomas in mice, in conjunction with ganciclovir administration. Jpn J Cancer Res. 1998 Jan;89(1):76–80. doi: 10.1111/j.1349-7006.1998.tb00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Csatary LK, Bakacs T. Use of Newcastle disease virus vaccine (MTH-68/H) in a patient with high-grade glioblastoma. Jama. 1999 May 5;281(17):1588–9. doi: 10.1001/jama.281.17.1588-a. [DOI] [PubMed] [Google Scholar]
- 29.Russell SJ. RNA viruses as virotherapy agents. Cancer Gene Ther. 2002 Dec;9(12):961–6. doi: 10.1038/sj.cgt.7700535. [DOI] [PubMed] [Google Scholar]
- 30.Ren H, Boulikas T, Lundstrom K, Soling A, Warnke PC, Rainov NG. Immunogene therapy of recurrent glioblastoma multiforme with a liposomally encapsulated replication-incompetent Semliki forest virus vector carrying the human interleukin-12 gene--a phase I/II clinical protocol. J Neurooncol. 2003 Aug-Sep;64(12):147–54. doi: 10.1007/BF02700029. [DOI] [PubMed] [Google Scholar]
- 31.Gridley DS, Andres ML, Li J, Timiryasova T, Chen B, Fodor I. Evaluation of radiation effects against C6 glioma in combination with vaccinia virus-p53 gene therapy. Int J Oncol. 1998 Nov;13(5):1093–8. doi: 10.3892/ijo.13.5.1093. [DOI] [PubMed] [Google Scholar]
- 32.Timiryasova TM, Chen B, Haghighat P, Fodor I. Vaccinia virus-mediated expression of wild-type p53 suppresses glioma cell growth and induces apoptosis. Int J Oncol. 1999 May;14(5):845–54. doi: 10.3892/ijo.14.5.845. [DOI] [PubMed] [Google Scholar]
- 33.Chen B, Timiryasova TM, Haghighat P, Andres ML, Kajioka EH, Dutta-Roy R, et al. Low-dose vaccinia virus-mediated cytokine gene therapy of glioma. J Immunother. 2001 Jan-Feb;24(1):46–57. doi: 10.1097/00002371-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 34.Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E. Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6803–8. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson CA, Cobbs C, Peduzzi JD, Novak M, Morrow CD. Repetitive intrathecal injections of poliovirus replicons result in gene expression in neurons of the central nervous system without pathogenesis. Hum Gene Ther. 2001 Oct 10;12(15):1827–41. doi: 10.1089/104303401753153893. [DOI] [PubMed] [Google Scholar]
- 36.Nabel GJ. Development of optimized vectors for gene therapy. Proc Natl Acad Sci U S A. 1999 Jan 19;96(2):324–6. doi: 10.1073/pnas.96.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergelson JM, Cunningham JA, Droguett G, Kurt-Jones EA, Krithivas A, Hong JS, et al. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science. 1997 Feb 28;275(5304):1320–3. doi: 10.1126/science.275.5304.1320. [DOI] [PubMed] [Google Scholar]
- 38.Tomko RP, Xu R, Philipson L. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci U S A. 1997 Apr 1;94(7):3352–6. doi: 10.1073/pnas.94.7.3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–19. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 40.Kim M, Sumerel LA, Belousova N, Lyons GR, Carey DE, Krasnykh V, et al. The coxsackievirus and adenovirus receptor acts as a tumour suppressor in malignant glioma cells. Br J Cancer. 2003 May 6;88(9):1411–6. doi: 10.1038/sj.bjc.6600932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998 Dec;72(12):9706–13. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J, et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res. 2002 Oct 15;62(20):5736–42. [PubMed] [Google Scholar]
- 43.Wickham TJ, Tzeng E, Shears LL, 2nd, Roelvink PW, Li Y, Lee GM, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997 Nov;71(11):8221–9. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Staba MJ, Wickham TJ, Kovesdi I, Hallahan DE. Modifications of the fiber in adenovirus vectors increase tropism for malignant glioma models. Cancer Gene Ther. 2000 Jan;7(1):13–9. doi: 10.1038/sj.cgt.7700104. [DOI] [PubMed] [Google Scholar]
- 45.Kawakami Y, Li H, Lam JT, Krasnykh V, Curiel DT, Blackwell JL. Substitution of the adenovirus serotype 5 knob with a serotype 3 knob enhances multiple steps in virus replication. Cancer Res. 2003 Mar 15;63(6):1262–9. [PubMed] [Google Scholar]
- 46.Takayama K, Reynolds PN, Short JJ, Kawakami Y, Adachi Y, Glasgow JN, et al. A mosaic adenovirus possessing serotype Ad5 and serotype Ad3 knobs exhibits expanded tropism. Virology. 2003 May 10;309(2):282–93. doi: 10.1016/s0042-6822(03)00067-9. [DOI] [PubMed] [Google Scholar]
- 47.Zheng S, Ulasov IV, Han Y, Tyler MA, Zhu ZB, Lesniak MS. Fiber-knob modifications enhance adenoviral tropism and gene transfer in malignant glioma. J Gene Med. 2007 Mar;9(3):151–60. doi: 10.1002/jgm.1008. [DOI] [PubMed] [Google Scholar]
- 48.Ulasov IV, Rivera AA, Han Y, Curiel DT, Zhu ZB, Lesniak MS. Targeting adenovirus to CD80 and CD86 receptors increases gene transfer efficiency to malignant glioma cells. J Neurosurg. 2007 Sep;107(3):617–27. doi: 10.3171/JNS-07/09/0617. [DOI] [PubMed] [Google Scholar]
- 49.Tsuruta Y, Pereboeva L, Glasgow JN, Luongo CL, Komarova S, Kawakami Y, et al. Reovirus sigma1 fiber incorporated into adenovirus serotype 5 enhances infectivity via a CAR-independent pathway. Biochem Biophys Res Commun. 2005 Sep 16;335(1):205–14. doi: 10.1016/j.bbrc.2005.07.054. [DOI] [PubMed] [Google Scholar]
- 50.Paul CP, Everts M, Glasgow JN, Dent P, Fisher PB, Ulasov IV, et al. Characterization of infectivity of knob-modified adenoviral vectors in glioma. Cancer Biol Ther. 2008 May;7(5):786–93. doi: 10.4161/cbt.7.5.5421. [DOI] [PubMed] [Google Scholar]
- 51.Perreau M, Mennechet F, Serratrice N, Glasgow JN, Curiel DT, Wodrich H, et al. Contrasting effects of human, canine, and hybrid adenovirus vectors on the phenotypical and functional maturation of human dendritic cells: implications for clinical efficacy. J Virol. 2007 Apr;81(7):3272–84. doi: 10.1128/JVI.01530-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sathornsumetee S, Rich JN. Designer therapies for glioblastoma multiforme. Ann N Y Acad Sci. 2008 Oct;1142:108–32. doi: 10.1196/annals.1444.009. [DOI] [PubMed] [Google Scholar]
- 53.van Beusechem VW, Mastenbroek DC, van den Doel PB, Lamfers ML, Grill J, Wurdinger T, et al. Conditionally replicative adenovirus expressing a targeting adapter molecule exhibits enhanced oncolytic potency on CAR-deficient tumors. Gene Ther. 2003 Nov;10(23):1982–91. doi: 10.1038/sj.gt.3302103. [DOI] [PubMed] [Google Scholar]
- 54.Wang W, Zhu NL, Chua J, Swenson S, Costa FK, Schmitmeier S, et al. Retargeting of adenoviral vector using basic fibroblast growth factor ligand for malignant glioma gene therapy. J Neurosurg. 2005 Dec;103(6):1058–66. doi: 10.3171/jns.2005.103.6.1058. [DOI] [PubMed] [Google Scholar]
- 55.Voelzke WR, Petty WJ, Lesser GJ. Targeting the epidermal growth factor receptor in high-grade astrocytomas. Curr Treat Options Oncol. 2008 Feb;9(1):23–31. doi: 10.1007/s11864-008-0053-5. [DOI] [PubMed] [Google Scholar]
- 56.Sonabend AM, Dana K, Lesniak MS. Targeting epidermal growth factor receptor variant III: a novel strategy for the therapy of malignant glioma. Expert Rev Anticancer Ther. 2007 Dec;7(12 Suppl):S45–50. doi: 10.1586/14737140.7.12s.S45. [DOI] [PubMed] [Google Scholar]
- 57.Frost E, Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- 58.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003 May 7;95(9):652–60. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 59.Manome Y, Wen PY, Chen L, Tanaka T, Dong Y, Yamazoe M, et al. Gene therapy for malignant gliomas using replication incompetent retroviral and adenoviral vectors encoding the cytochrome P450 2B1 gene together with cyclophosphamide. Gene Ther. 1996 Jun;3(6):513–20. [PubMed] [Google Scholar]
- 60.Chen J, Bezdek T, Chang J, Kherzai AW, Willingham T, Azzara M, et al. A glial-specific, repressible, adenovirus vector for brain tumor gene therapy. Cancer Res. 1998 Aug 15;58(16):3504–7. [PubMed] [Google Scholar]
- 61.Lammering G, Hewit TH, Valerie K, Lin PS, Contessa JN, Schmidt-Ullrich RK. Anti-erbB receptor strategy as a gene therapeutic intervention to improve radiotherapy in malignant human tumours. Int J Radiat Biol. 2003 Jul;79(7):561–8. doi: 10.1080/0955300031000102632. [DOI] [PubMed] [Google Scholar]
- 62.Lammering G, Lin PS, Contessa JN, Hampton JL, Valerie K, Schmidt-Ullrich RK. Adenovirus-mediated overexpression of dominant negative epidermal growth factor receptor-CD533 as a gene therapeutic approach radiosensitizes human carcinoma and malignant glioma cells. Int J Radiat Oncol Biol Phys. 2001 Nov 1;51(3):775–84. doi: 10.1016/s0360-3016(01)01714-x. [DOI] [PubMed] [Google Scholar]
- 63.Su ZZ, Lebedeva IV, Sarkar D, Gopalkrishnan RV, Sauane M, Sigmon C, et al. Melanoma differentiation associated gene-7, mda-7/IL-24, selectively induces growth suppression, apoptosis and radiosensitization in malignant gliomas in a p53-independent manner. Oncogene. 2003 Feb 27;22(8):1164–80. doi: 10.1038/sj.onc.1206062. [DOI] [PubMed] [Google Scholar]
- 64.Smitt PS, Driesse M, Wolbers J, Kros M, Avezaat C. Treatment of relapsed malignant glioma with an adenoviral vector containing the herpes simplex thymidine kinase gene followed by ganciclovir. Mol Ther. 2003 Jun;7(6):851–8. doi: 10.1016/s1525-0016(03)00100-x. [DOI] [PubMed] [Google Scholar]
- 65.Kang YA, Shin HC, Yoo JY, Kim JH, Kim JS, Yun CO. Novel cancer antiangiotherapy using the VEGF promoter-targeted artificial zinc-finger protein and oncolytic adenovirus. Mol Ther. 2008 Jun;16(6):1033–40. doi: 10.1038/mt.2008.63. [DOI] [PubMed] [Google Scholar]
- 66.Zhang WW, Alemany R, Wang J, Koch PE, Ordonez NG, Roth JA. Safety evaluation of Ad5CMV-p53 in vitro and in vivo. Hum Gene Ther. 1995 Feb;6(2):155–64. doi: 10.1089/hum.1995.6.2-155. [DOI] [PubMed] [Google Scholar]
- 67.Lang FF, Yung WK, Sawaya R, Tofilon PJ. Adenovirus-mediated p53 gene therapy for human gliomas. Neurosurgery. 1999 Nov;45(5):1093–104. doi: 10.1097/00006123-199911000-00016. [DOI] [PubMed] [Google Scholar]
- 68.Vecil GG, Lang FF. Clinical trials of adenoviruses in brain tumors: a review of Ad-p53 and oncolytic adenoviruses. J Neurooncol. 2003 Dec;65(3):237–46. doi: 10.1023/b:neon.0000003653.45635.32. [DOI] [PubMed] [Google Scholar]
- 69.Lang FF, Bruner JM, Fuller GN, Aldape K, Prados MD, Chang S, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003 Jul 1;21(13):2508–18. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 70.Kuriyama N, Kuriyama H, Julin CM, Lamborn K, Israel MA. Pretreatment with protease is a useful experimental strategy for enhancing adenovirus-mediated cancer gene therapy. Hum Gene Ther. 2000 Nov 1;11(16):2219–30. doi: 10.1089/104303400750035744. [DOI] [PubMed] [Google Scholar]
- 71.Kim JH, Lee YS, Kim H, Huang JH, Yoon AR, Yun CO. Relaxin expression from tumor-targeting adenoviruses and its intratumoral spread, apoptosis induction, and efficacy. J Natl Cancer Inst. 2006 Oct 18;98(20):1482–93. doi: 10.1093/jnci/djj397. [DOI] [PubMed] [Google Scholar]
- 72.Mok W, Boucher Y, Jain RK. Matrix metalloproteinases-1 and -8 improve the distribution and efficacy of an oncolytic virus. Cancer Res. 2007 Nov 15;67(22):10664–8. doi: 10.1158/0008-5472.CAN-07-3107. [DOI] [PubMed] [Google Scholar]
- 73.Trask TW, Trask RP, Aguilar-Cordova E, Shine HD, Wyde PR, Goodman JC, et al. Phase I study of adenoviral delivery of the HSV-tk gene and ganciclovir administration in patients with current malignant brain tumors. Mol Ther. 2000 Feb;1(2):195–203. doi: 10.1006/mthe.2000.0030. [DOI] [PubMed] [Google Scholar]
- 74.Sandmair AM, Loimas S, Puranen P, Immonen A, Kossila M, Puranen M, et al. Thymidine kinase gene therapy for human malignant glioma, using replication-deficient retroviruses or adenoviruses. Hum Gene Ther. 2000 Nov 1;11(16):2197–205. doi: 10.1089/104303400750035726. [DOI] [PubMed] [Google Scholar]
- 75.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003 Dec;65(3):279–89. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 76.Immonen A, Vapalahti M, Tyynela K, Hurskainen H, Sandmair A, Vanninen R, et al. AdvHSV-tk gene therapy with intravenous ganciclovir improves survival in human malignant glioma: a randomised, controlled study. Mol Ther. 2004 Nov;10(5):967–72. doi: 10.1016/j.ymthe.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Wildner O, Morris JC, Vahanian NN, Ford H, Jr, Ramsey WJ, Blaese RM. Adenoviral vectors capable of replication improve the efficacy of HSVtk/GCV suicide gene therapy of cancer. Gene Ther. 1999 Jan;6(1):57–62. doi: 10.1038/sj.gt.3300810. [DOI] [PubMed] [Google Scholar]
- 78.Nanda D, Vogels R, Havenga M, Avezaat CJ, Bout A, Smitt PS. Treatment of malignant gliomas with a replicating adenoviral vector expressing herpes simplex virus-thymidine kinase. Cancer Res. 2001 Dec 15;61(24):8743–50. [PubMed] [Google Scholar]
- 79.Sonabend AM, Ulasov IV, Lesniak MS. Conditionally replicative adenoviral vectors for malignant glioma. Rev Med Virol. 2006 Mar-Apr;16(2):99–115. doi: 10.1002/rmv.490. [DOI] [PubMed] [Google Scholar]
- 80.Heise C, Sampson-Johannes A, Williams A, McCormick F, Von Hoff DD, Kirn DH. ONYX-015, an E1B gene-attenuated adenovirus, causes tumor-specific cytolysis and antitumoral efficacy that can be augmented by standard chemotherapeutic agents. Nat Med. 1997 Jun;3(6):639–45. doi: 10.1038/nm0697-639. [DOI] [PubMed] [Google Scholar]
- 81.Fueyo J, Gomez-Manzano C, Alemany R, Lee PS, McDonnell TJ, Mitlianga P, et al. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000 Jan 6;19(1):2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 82.Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R. A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res. 2001 Jan;7(1):120–6. [PubMed] [Google Scholar]
- 83.Miller CR, Buchsbaum DJ, Reynolds PN, Douglas JT, Gillespie GY, Mayo MS, et al. Differential susceptibility of primary and established human glioma cells to adenovirus infection: targeting via the epidermal growth factor receptor achieves fiber receptor-independent gene transfer. Cancer Res. 1998 Dec 15;58(24):5738–48. [PubMed] [Google Scholar]
- 84.van Beusechem VW, Grill J, Mastenbroek DC, Wickham TJ, Roelvink PW, Haisma HJ, et al. Efficient and selective gene transfer into primary human brain tumors by using single-chain antibody-targeted adenoviral vectors with native tropism abolished. J Virol. 2002 Mar;76(6):2753–62. doi: 10.1128/JVI.76.6.2753-2762.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohno S, Nakagawa K, Hamada K, Harada H, Yamasaki K, Hashimoto K, et al. Midkine promoter-based conditionally replicative adenovirus for malignant glioma therapy. Oncol Rep. 2004 Jul;12(1):73–8. [PubMed] [Google Scholar]
- 86.Parr MJ, Manome Y, Tanaka T, Wen P, Kufe DW, Kaelin WG, Jr, et al. Tumor-selective transgene expression in vivo mediated by an E2F-responsive adenoviral vector. Nat Med. 1997 Oct;3(10):1145–9. doi: 10.1038/nm1097-1145. [DOI] [PubMed] [Google Scholar]
- 87.Shinoura N, Saito K, Yoshida Y, Hashimoto M, Asai A, Kirino T, et al. Adenovirus-mediated transfer of bax with caspase-8 controlled by myelin basic protein promoter exerts an enhanced cytotoxic effect in gliomas. Cancer Gene Ther. 2000 May;7(5):739–48. doi: 10.1038/sj.cgt.7700158. [DOI] [PubMed] [Google Scholar]
- 88.Vandier D, Rixe O, Besnard F, Kim M, Rikiyama T, Goldsmith M, et al. Inhibition of glioma cells in vitro and in vivo using a recombinant adenoviral vector containing an astrocyte-specific promoter. Cancer Gene Ther. 2000 Aug;7(8):1120–6. doi: 10.1038/sj.cgt.7700211. [DOI] [PubMed] [Google Scholar]
- 89.McCormick F. Interactions between adenovirus proteins and the p53 pathway: the development of ONYX-015. Semin Cancer Biol. 2000 Dec;10(6):453–9. doi: 10.1006/scbi.2000.0336. [DOI] [PubMed] [Google Scholar]
- 90.Heise CC, Williams AM, Xue S, Propst M, Kirn DH. Intravenous administration of ONYX-015, a selectively replicating adenovirus, induces antitumoral efficacy. Cancer Res. 1999 Jun 1;59(11):2623–8. [PubMed] [Google Scholar]
- 91.Chiocca EA, Abbed KM, Tatter S, Louis DN, Hochberg FH, Barker F, et al. A phase I open-label, dose-escalation, multi-institutional trial of injection with an E1B-Attenuated adenovirus, ONYX-015, into the peritumoral region of recurrent malignant gliomas, in the adjuvant setting. Mol Ther. 2004 Nov;10(5):958–66. doi: 10.1016/j.ymthe.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 92.Alonso MM, Cascallo M, Gomez-Manzano C, Jiang H, Bekele BN, Perez-Gimenez A, et al. ICOVIR-5 shows E2F1 addiction and potent antiglioma effect in vivo. Cancer Res. 2007 Sep 1;67(17):8255–63. doi: 10.1158/0008-5472.CAN-06-4675. [DOI] [PubMed] [Google Scholar]
- 93.New PZ, Baskin D, Trask T, Cavaliere R, Chaudhury AR, Bell S, et al. Radiographic and immunologic responses to adjuvant immunotherapy for malignant gliomas. J Clin Oncol (Meeting Abstracts) 2008 May 20;26(15suppl):2039. [Google Scholar]
- 94.Miyao Y, Shimizu K, Tamura M, Akita H, Ikeda K, Mabuchi E, et al. Usefulness of a mouse myelin basic protein promoter for gene therapy of malignant glioma: myelin basic protein promoter is strongly active in human malignant glioma cells. Jpn J Cancer Res. 1997 Jul;88(7):678–86. doi: 10.1111/j.1349-7006.1997.tb00436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Boyd M, Mairs RJ, Mairs SC, Wilson L, Livingstone A, Cunningham SH, et al. Expression in UVW glioma cells of the noradrenaline transporter gene, driven by the telomerase RNA promoter, induces active uptake of [131I]MIBG and clonogenic cell kill. Oncogene. 2001 Nov 22;20(53):7804–8. doi: 10.1038/sj.onc.1204955. [DOI] [PubMed] [Google Scholar]
- 96.Komata T, Kondo Y, Kanzawa T, Ito H, Hirohata S, Koga S, et al. Caspase-8 gene therapy using the human telomerase reverse transcriptase promoter for malignant glioma cells. Hum Gene Ther. 2002 Jun 10;13(9):1015–25. doi: 10.1089/104303402753812421. [DOI] [PubMed] [Google Scholar]
- 97.Kajiwara Y, Yamasaki F, Hama S, Yahara K, Yoshioka H, Sugiyama K, et al. Expression of survivin in astrocytic tumors: correlation with malignant grade and prognosis. Cancer. 2003 Feb 15;97(4):1077–83. doi: 10.1002/cncr.11122. [DOI] [PubMed] [Google Scholar]
- 98.Kleinschmidt-DeMasters BK, Heinz D, McCarthy PJ, Bobak JB, Lillehei KO, Shroyer AL, et al. Survivin in glioblastomas. Protein and messenger RNA expression and comparison with telomerase levels. Arch Pathol Lab Med. 2003 Jul;127(7):826–33. doi: 10.5858/2003-127-826-SIG. [DOI] [PubMed] [Google Scholar]
- 99.Ulasov IV, Rivera AA, Sonabend AM, Rivera LB, Wang M, Zhu ZB, et al. Comparative evaluation of survivin, midkine and CXCR4 promoters for transcriptional targeting of glioma gene therapy. Cancer Biol Ther. 2007 May;6(5):679–85. doi: 10.4161/cbt.6.5.3957. [DOI] [PubMed] [Google Scholar]
- 100.Zhu ZB, Makhija SK, Lu B, Wang M, Rivera AA, Kim-Park S, et al. Incorporating the survivin promoter in an infectivity enhanced CRAd-analysis of oncolysis and anti-tumor effects in vitro and in vivo. Int J Oncol. 2005 Jul;27(1):237–46. [PubMed] [Google Scholar]
- 101.Van Houdt WJ, Haviv YS, Lu B, Wang M, Rivera AA, Ulasov IV, et al. The human survivin promoter: a novel transcriptional targeting strategy for treatment of glioma. J Neurosurg. 2006 Apr;104(4):583–92. doi: 10.3171/jns.2006.104.4.583. [DOI] [PubMed] [Google Scholar]
- 102.Ulasov IV, Zhu ZB, Tyler MA, Han Y, Rivera AA, Khramtsov A, et al. Survivin-driven and fiber-modified oncolytic adenovirus exhibits potent antitumor activity in established intracranial glioma. Hum Gene Ther. 2007 Jul;18(7):589–602. doi: 10.1089/hum.2007.002. [DOI] [PubMed] [Google Scholar]
- 103.Nandi S, Ulasov IV, Tyler MA, Sugihara AQ, Molinero L, Han Y, et al. Low-dose radiation enhances survivin-mediated virotherapy against malignant glioma stem cells. Cancer Res. 2008 Jul 15;68(14):5778–84. doi: 10.1158/0008-5472.CAN-07-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wohlfahrt ME, Beard BC, Lieber A, Kiem HP. A capsid-modified, conditionally replicating oncolytic adenovirus vector expressing TRAIL Leads to enhanced cancer cell killing in human glioblastoma models. Cancer Res. 2007 Sep 15;67(18):8783–90. doi: 10.1158/0008-5472.CAN-07-0357. [DOI] [PubMed] [Google Scholar]
- 105.Dent P, Yacoub A, Park M, Sarkar D, Shah K, Curiel DT, et al. Searching for a cure: gene therapy for glioblastoma. Cancer Biol Ther. 2008 Sep;7(9):1335–40. doi: 10.4161/cbt.7.9.6408. [DOI] [PubMed] [Google Scholar]
- 106.Ulasov IV, Sonabend AM, Nandi S, Khramtsov A, Han Y, Lesniak MS. Combination of adenoviral virotherapy and temozolomide chemotherapy eradicates malignant glioma through autophagic and apoptotic cell death in vivo. Br J Cancer. 2009 Apr 7;100(7):1154–64. doi: 10.1038/sj.bjc.6604969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sonabend AM, Ulasov IV, Tyler MA, Rivera AA, Mathis JM, Lesniak MS. Mesenchymal stem cells effectively deliver an oncolytic adenovirus to intracranial glioma. Stem Cells. 2008 Mar;26(3):831–41. doi: 10.1634/stemcells.2007-0758. [DOI] [PubMed] [Google Scholar]
- 108.Tyler MA, Ulasov IV, Sonabend AM, Nandi S, Han Y, Marler S, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009 Feb;16(2):262–78. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Aboody KS, Najbauer J, Danks MK. Stem and progenitor cell-mediated tumor selective gene therapy. Gene Ther. 2008 May;15(10):739–52. doi: 10.1038/gt.2008.41. [DOI] [PubMed] [Google Scholar]
- 110.Donson AM, Foreman NK. Adenovirus mediated gene therapy in a glioblastoma vaccine model; specific antitumor immunity and abrogation of immunosuppression. J Neurooncol. 1998 Dec;40(3):205–14. doi: 10.1023/a:1006106026317. [DOI] [PubMed] [Google Scholar]
- 111.Munz C, Naumann U, Grimmel C, Rammensee HG, Weller M. TGF-beta-independent induction of immunogenicity by decorin gene transfer in human malignant glioma cells. Eur J Immunol. 1999 Mar;29(3):1032–40. doi: 10.1002/(SICI)1521-4141(199903)29:03<1032::AID-IMMU1032>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 112.Wakimoto H, Fulci G, Tyminski E, Chiocca EA. Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide’s enhancement of viral oncolysis. Gene Ther. 2004 Jan;11(2):214–23. doi: 10.1038/sj.gt.3302143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lamfers ML, Fulci G, Gianni D, Tang Y, Kurozumi K, Kaur B, et al. Cyclophosphamide increases transgene expression mediated by an oncolytic adenovirus in glioma-bearing mice monitored by bioluminescence imaging. Mol Ther. 2006 Dec;14(6):779–88. doi: 10.1016/j.ymthe.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiocca EA, Smith KM, McKinney B, Palmer CA, Rosenfeld S, Lillehei K, et al. A phase I trial of Ad.hIFN-beta gene therapy for glioma. Mol Ther. 2008 Mar;16(3):618–26. doi: 10.1038/sj.mt.6300396. [DOI] [PubMed] [Google Scholar]
- 115.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005 Feb 15;105(4):1815–22. doi: 10.1182/blood-2004-04-1559. [DOI] [PubMed] [Google Scholar]
- 116.Krampera M, Pasini A, Rigo A, Scupoli MT, Tecchio C, Malpeli G, et al. HB-EGF/HER-1 signaling in bone marrow mesenchymal stem cells: inducing cell expansion and reversibly preventing multilineage differentiation. Blood. 2005 Jul 1;106(1):59–66. doi: 10.1182/blood-2004-09-3645. [DOI] [PubMed] [Google Scholar]
- 117.Rasmusson I, Ringden O, Sundberg B, Le Blanc K. Mesenchymal stem cells inhibit lymphocyte proliferation by mitogens and alloantigens by different mechanisms. Exp Cell Res. 2005 Apr 15;305(1):33–41. doi: 10.1016/j.yexcr.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 118.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005 Sep 1;106(5):1755–61. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 119.Spaggiari GM, Capobianco A, Becchetti S, Mingari MC, Moretta L. Mesenchymal stem cell-natural killer cell interactions: evidence that activated NK cells are capable of killing MSCs, whereas MSCs can inhibit IL-2-induced NK-cell proliferation. Blood. 2006 Feb 15;107(4):1484–90. doi: 10.1182/blood-2005-07-2775. [DOI] [PubMed] [Google Scholar]


