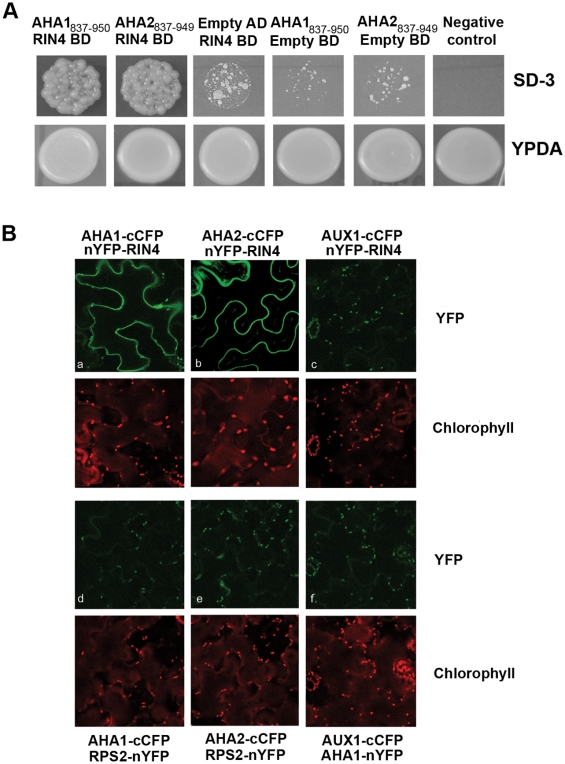
Figure 1. RIN4 and AHA interact in planta and in yeast.
(A) RIN4 interacts with the C terminus of both AHA1 and AHA2 using the Matchmaker yeast two-hybrid system (Clontech). BD, binding domain vector (pGBKT7); AD, activation domain vector (pGADT7); SD-3, synthetic dextrose media lacking leucine, tryptophan, and histidine; YPDA, yeast potato dextrose agar. AHA1 and AHA2 were cloned into the AD vector. (B) AHA1 and AHA2 associate with RIN4 in vivo. We were able to detect a specific interaction between AHA1 and AHA2 with RIN4 by BiFC across three replications. The experiments to detect BiFC fluorescence with AHA1 and AHA2 were conducted independently. N. benthamiana leaves co-expressing either AHA1 or AHA2 and RIN4 results in detectable GFP fluorescence on the membrane (upper panel, a–b). No interaction with RPS2 (lower panel, d–e) or AUX1 (c, f) could be detected. YFP fluorescence was excited at 488 and imaged at 518–540 nm by confocal microscopy, except for b and e (used excitation 512 and emission 525–540 nm). Chlorophyll emission was detected at 618 nm.