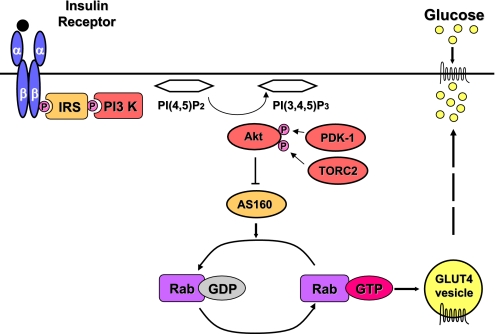
Fig. 1.
Schematic illustration of insulin-stimulated GLUT4 translocation. Binding of insulin (black circle) to its tetrameric receptor (2 α- and 2 β-subunits) leads to tyrosine phosphorylation (P) of insulin receptor substrate (IRS) proteins and the recruitment of phosphatidylinositol 3 kinase (PI 3-kinase), which catalyzes the conversion of PI(4,5)P2 to PI(3,4,5)P3. PI(3,4,5)P3 generation results in the phosphorylation of AKT2 (protein kinase B, beta) by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and transducer of regulated cAMP response element-binding protein 2 (TORC2), causing its activation. AKT2 phosphorylates TBC1D4 (AS160) suppressing its GAP activity and resulting in active GTP-bound Rab proteins that promote glucose transporter 4 (GLUT4) vesicle translocation. PI(4,5)P2, phosphotidylinositol 4,5 bisphosphate; PI(3,4,5)P3, phosphotidylinositol 3,4,5 trisphosphate; GEF, guanine exchange factor; GTP, guanosine triphosphate; GDP, guanosine diphosphate; TBC1D4, Tre-2, BUB2, CDC16, 1 domain family member 4 (also known as AS160, AKT substrate of 160 kDa.