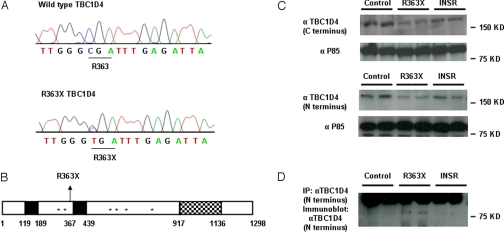
Fig. 2.
Identification of the R363X TBC1D4 mutation. (A) Wild-type TBC1D4 and R363X TBC1D4 mutant sequence traces. The heterozygous substitution of thymine for cytosine results in the substitution of a stop codon for arginine (R) at amino acid 363. (B) Schematic representation of TBC1D4 showing the position of the R363X premature stop mutation, phosphotyrosine binding domains (PTB, solid black regions), AKT phosphorylation sites (*), and RAB GTPase activating protein (GAP) domain of TBC1D4 (checked region). (C) TBC1D4 expression levels (160 kDa) as determined by immunoblot analysis with both a C-terminal and N-terminal TBC1D4 antibody in EBVL lysates from the proband (R363X) compared with protein expression in cells from one healthy control (control) and one severely insulin-resistant subject with a loss-of-function mutation in the INSR gene (INSR). Τhe lysates were reprobed with an antibody raised against the P85 subunit of PI3K to confirm equal loading. (D) Expression of the truncated TBC1D4 protein in EBVL lysates from the proband confirmed by immunoprecipitation (IP) and subsequent immunoblotting with an N-terminal TBC1D4 antibody. The unexpectedly large size of the truncated protein (about 80 kDa) is the result of the slower mobility of the nonreduced form (see Fig. S1).