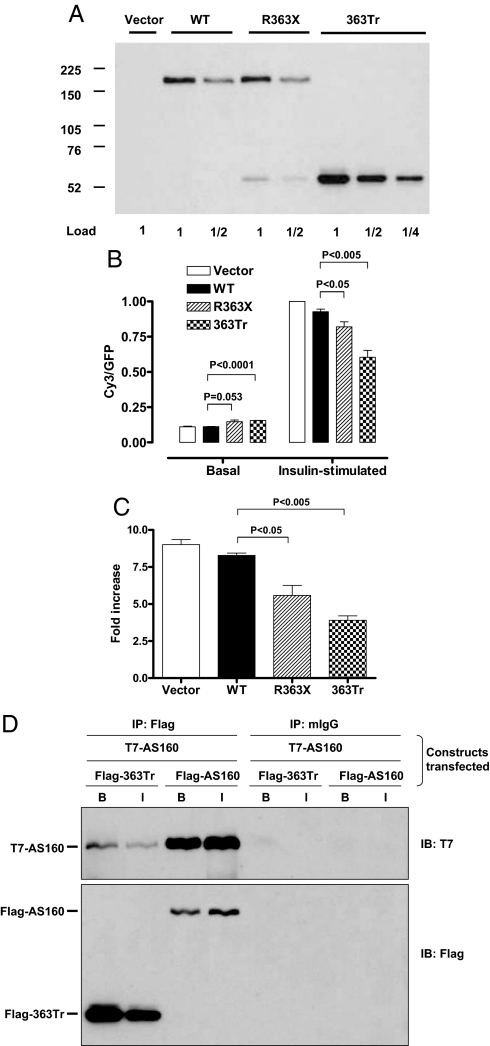
Fig. 3.
Cellular analysis of GLUT4 translocation. (A) In vitro expression levels of TBC1D4 protein in 3T3L1 adipocytes (full-length TBC1D4 at 160 kDa and truncated protein at 52 kDa) transfected with empty P3XFLAG CMV10 vector (vector), wild-type TBC1D4 vector (WT), R363X vector (R363X), or the artificially generated 363 truncated protein (363Tr) vector as determined by immunoblotting with anti-Flag. We have also immunoblotted these samples with an antibody against a conserved peptide in mouse and human TBC1D4. Since the ectopic human TBC1D4 has a slightly lower mobility than the endogenous mouse TBC1D4, it was possible to estimate the relative amounts of each; the ectopic wild-type and R363X full length TBC1D4 were ≈10-fold more abundant than the endogenous TBC1D4 (data not shown). Also then by comparison of the anti-Flag signals of full-length R363X and 363Tr, we estimate that the 363Tr was overexpressed by about 30-fold in the transfected cells, relative to endogenous TBC1D4. (B) Cell surface GLUT4 levels, normalized to a value of 1.0 for the vector control in the insulin-stimulated state. Values are expressed as means ± SE for 4–6 independent measurements of the Cy3/GFP ratio in cells with and without 30-min treatment with 160 nM of insulin. (C) Data from Fig. 2B expressed as the ratio of cell surface GLUT4 in the insulin-stimulatd state to that in the basal state. (D) Immunoblots (IB) showing coimmunoprecipitation (IP) of T7-tagged TBC1D4 from basal (B) and insulin-stimulated (I) 293 cells with Flag-tagged 363Tr or full-length TBC1D4. To control for nonspecific immunoprecipitation, lysates were also immunoblotted after immunoprecipitation with irrelevant mouse Ig and protein G-agarose (mIgG).