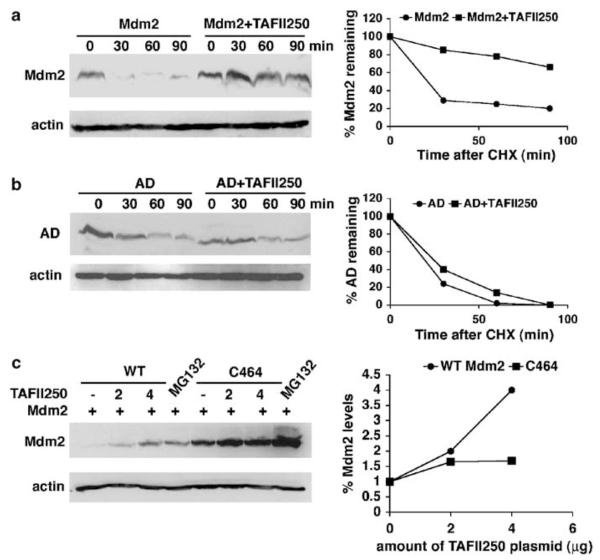
Figure 5.
TAFII250 stabilizes Mdm2. (a) Mdm2 half-life was measured in the presence and absence of TAFII250. H1299 cells were transfected with 5 μg of Mdm2 expression vector, with 5 μg of TAFII250 expression vector or empty vector. At 36 h post-transfection the cells were treated with 10 μg/ml of cycloheximide and harvested at different time points as indicated. Extracts were analysed by Western blotting for the expression levels of Mdm2 using SMP14 and D12 antibodies. The intensities of the signals were quantified and represented in a graph (right). (b) The acidic domain is important for the stabilization of Mdm2 by TAFII250. H1299 cells were transfected with 5 μg ‘AD’ encoding Mdm2 that contains a deletion in the acidic domain (222–270 aa), together with TAFII250 (5 μg) expression plasmid or with empty vector (5 μg). The densities of the Mdm2 signals were quantified and represented in the graph on the right. (c) H1299 cells were transfected with plasmids expressing wild-type Mdm2 or the C464 mutant (2 μg) together with increasing amounts of TAFII250 expression vector (0, 2, 4 μg). One plate of cells transfected with Mdm2 and one transfected with C464 were treated with 20 μM of MG132 for 4 h. Cells were harvested and lysates were analysed by Western blotting with anti-Mdm2 antibody (SMP14 and D12).