Abstract
Hemiasterlin (Hem) and dolastatin (Dol) are marine natural products which are cytotoxic for cancer cells. Hem, a tripeptide, and Dol, a hexapeptide, were conjugated with linkers (L) to the universal BB agonist DPhe-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2(BA1) and the effects of the Hem-BB and Dol-BB conjugates investigated on NCI-H1299 lung cancer cells. Hem-LA-BA1 and Hem-LB-BA1 inhibited specific (125I-Tyr4)BB binding to NCI-H1299 cells, which have BB2 receptors (R), with IC50 values of 15 and 25 nM, respectively. Addition of Hem-LA-BA1 and Hem-LB-BA1 to Fura-2 AM loaded cells containing BB2R, caused elevated cytosolic Ca2+. In a growth assay, Hem-LA-BA1 and Hem-LB-BA1 inhibited the proliferation of NCI-H1299 cells. Dol-succinamide (Dols)-LD-BA1 and Dols-LE-BA1 bound with high affinity to NCI-H1299 cells and elevated cytosolic Ca2+, but did not inhibit the proliferation of NCI-H1299 cells. Also, Hem-LA-BA1 inhibited 125I-DTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2 (BA2) binding to Balb/3T3 cells transfected with BB1R or BB2R as well as with BRS-3 with IC50 values of 130, 8, and 540 nM, respectively. These results show that Hem-BB conjugates are cytotoxic for cancer cells containing BB2R.
Keywords: bombesin, dolastatin, hemiasterlin, lung cancer
Introduction
Marine natural products have been used for the treatment of diseases such as acquired immuno-deficiency disease (Rawat et al., 2006). Marine natural products have been isolated from sponges, moluscs, bryozoans as well as tunicates and range in structure from linear peptides to complex macrocyclic polyethers. Two such peptides include hemiasterlin (Hem), which is isolated from the South African sponge (Talpir et al., 1994) and dolastatin (Dol), which is isolated from the Indian Ocean mollusk (Pettit et al, 1989). Hem and its analog HTI-286 inhibit the proliferation of tumor cells during mitosis by interaction with tubulin (Krishnamurthy et al. 2003). Similarly, Dol and its analog TZT-1027 interact with tubulin and are cytotoxic for cancer cells (Kobayashi et al., 1997). Dol induces G2/M cell cycle arrest and causes apoptosis of cancer cells via mitochondrial and Fas mediated pathways (Sato et al., 2007). Both Hem and Dol are being evaluated in phase I and phase II clinical trials using cancer patients. In some clinical trials, however, Dol 10 had toxic side-effects including peripheral neuropathy (Pitot et al., 1999). This toxicity might be decreased if marine toxins can be delivered using molecular targets associated with the cancer cells.
Bombesin/gastrin releasing peptide (BB/GRP) is an autocrine growth factor for some small cell lung cancer cells (SCLC) (Moody et al., 1981, Wood et al., 1981, Cuttitta et al., 1985). Lung cancer, which kills over 160,000 U.S. citizens annually, is traditionally treated with chemo- and/or radiation therapy (Sekido et al., 2005), however, relapse frequently occurs and the median survival time is under 1 year. BB binds with high affinity to a G-protein coupled receptor, the BB2 receptor (Battey et al., 1991, Spindel et al., 1990). Two other receptors of this family include the neuromedin B receptor, the BB1 receptor (Wada et al., 1991), and BB receptor subtype-3 (BRS-3) (Fathi et al., 1993). BB receptors are present in over 50% of the tumors derived from lung cancer, breast cancer, prostate cancer, head/neck squamous cell cancer, glioblastoma and neuroblastoma (Jensen and Moody, 2006). These BB receptors may serve as molecular targets to develop new drugs for treatment of lung cancer patients.
Prodrugs decrease drug associated side-effects and enhance tumor cytotoxicity (deGroot et al., 2001). One group of prodrugs uses ligands for peptide growth factor receptors that are overexpressed in cancer cells. BB, luteinizing hormone-releasing hormone, somatostatin and vasoactive intestinal peptide analogs have been coupled to cytotoxic agents and have potent antitumor effects (Buchholz et al., 2006; Nagy and Schally, 2005). These peptide-chemotherapeutic conjugates are delivered to the cancer cell by receptor-mediated endocytosis and the cytotoxic agent released into the cancer cell after enzymatic catalysis. Previously, we developed BB-camptothecin (CPT) conjugates which bound with high affinity to BB1R and BB2R as well as BRS-3 (Moody et al., 2004). BB receptors are present in many lung cancer tumors (Reubi et al., 2002). The BB-CPT conjugates were readily internalized by lung cancer cells containing BB1R, BB2R as well as BRS-3 (Moody et al., 2006). The BB-CPT conjugates were metabolized by P450 enzymes releasing the cytotoxic CPT, which is a topoisomerase 1 inhibitor, leading to cytotoxicity of the lung cancer cells (Patterson et al., 1999).
In this communication Hem or Dol were coupled to universal agonist DPhe-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2 (BA1), which binds with high affinity to all classes of mammalian BB receptors (Mantey et al., 1997; Pradhan et al., 1998), and their effects tested in NCI-H1299 lung cancer cells. Hem-BB and Dol-BB conjugates bound with high affinity to NCI-H1299 lung cancer cells which have BB2R (IC50 values 15-150 nM). Also, the Hem-BB and Dol-BB conjugates bound with high affinity to Balb 3T3 cells transfected with BB2R and caused elevation of cytosolic Ca2+. The Hem-BB, but not Dol-BB conjugates, inhibited the growth of NCI-H1299 cells. These results indicate that Hem-BB conjugates are cytotoxic for lung cancer cells which have BB receptors.
Materials and Methods
Cell culture
NCI-H1299 cells were cultured in RPMI-1640 medium containing 10% heat inactivated fetal bovine serum (FBS). NCI-H1299 cells were split weekly 1/20 with trypsin-EDTA. Balb 3T3 cells stably expressing human BRS-3, BB1R or BB2R (Benya et al., 1995) were grown in DMEM supplemented with 300 mg/l G418 sulfate. Cells were mycoplasm free and were used when they were in exponential growth phase after incubation at 37°C in 5% CO2 and 95% air.
Receptor binding
(125I-Tyr4)BB and 125I-DTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2 (125I-BA2) with specific activities of 2200 Ci/mmol were prepared as described (Moody et al., 2004). The radiolabeled peptides were separated using a C18 Sep-Pak (Waters Associates, Milford, MA) and further purified by reverse-phase high pressure liquid chromatography on a C18 column. The fractions with the highest radioactivity were pooled, neutralized with 0.2 N Tris buffer, pH 9.5, and stored with 0.5% (w/v) bovine serum albumin (BSA) at -20°C. Binding was performed using human lung cancer NCI-H1299 cells or Balb 3T3 cells stably expressing BB2R (0.3 × 106/cell), BB1R (0.03 × 106/cell) or BRS-3 (0.3 × 106/cell). A monolayer of cells in 24 well plates was incubated with 0.05 nM 125I-BA2 in PBS containing 0.25% BSA and 250 ug/ml bacitracin at 37°C for 20 min or 25°C for 60 min. The nonsaturable binding was the amount of radioactivity associated with cells in incubations containing 0.05 nM radioligand (2200 Ci/mmol) and 1000 nM unlabeled BA2. Nonsaturable binding was <10% of total binding in all the experiments. Inhibition constants (Ki) were determined using a least-square, curve-fitting program (KaleidaGraph) and the Cheng-Prusoff equation (Cheng and Prusoff, 1973).
Peptide synthesis
Linker(L)-BA1 peptides were synthesized using solid phase techniques. Hem and Dol peptides were synthesized with a Fmoc N-terminal and free carboxyl group. The C-terminal of Hem and Dol were then coupled to the N-terminal of L-BA1 using carbodiimide. The N-terminal of the products was deprotected and Hem-LA-BA1, Hem-LB-BA1 and Dol-LC-BA1 cleaved from the resin (Fig. 1). In addition, solid phase L-BA1 peptides were coupled to Dol in which the N-terminal was succinnylated (Dols). Dols-LD-BA1 and Dols-LE-BA1 were cleaved from the resin (Fig. 1). The molecular weights of Hem-LA-BA1, Hem-LB-BA1, Dol-LC-BA1, Dols-LD-BA1 and Dols-LE-BA1 were 2570, 2183, 2725, 1894 and 2320 Daltons, respectively. Peptide purity was greater than 98% based on HPLC, amino acid and mass spectroscopy analysis.
Figure 1.
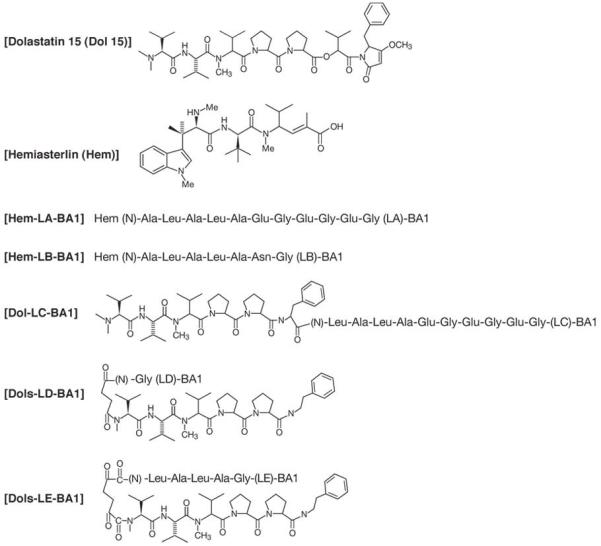
Structures of peptides. The chemical structures of hemiasterlin, dolastatin-15, Dol and Dols are shown. The peptide structures of LA, LB, LC, LD and LE are shown. (N) represents the N-terminal.
Cytosolic Calcium [Ca2+]i
The ability of the Dol-BB and Hem-BB conjugates to alter cytosolic [Ca2+]i was investigated as described previously (Moody et al., 1987). NCI-H1299 cells and Balb/3T3 cells containing BB2R were harvested (2.5 × 106/ml) and incubated with 5 μM Fura 2 AM at 37°C for 30 min. The cells, which contained loaded Fura 2, were centrifuged at 1500 x g for 10 min and resuspended at the same concentration in SIT medium (RPMI-1640 containing 30 nM sodium selenite, 5 μg/ml bovine insulin and 10 μg/ml transferrin). The cells were placed a cuvette and analyzed using a Delta PTI Scan 1 spectrofluorometer (Photon Technology International, South Brunswick, NJ) equipped with a magnetic stirring mechanism and temperature-regulated cuvette holder. The fluorescence intensity was continuously monitored at dual excitation wavelengths of 340 nm and 380 nm, using an emission wavelength of 510 nm prior to and after the addition of BB-like peptides.
Proliferation Assays
Growth studies in vitro were conducted using the [3-(4,5 dimethylthiazol-2-yl)-2.5-diphenyl-2H-tetrazolium bromide] (MTT) (Sigma Chemical Co., St. Louis, MO) colorimetric assays. NCI-H1299 cells were placed in 100 ul of SIT medium and various concentrations of Hem-BB or Dol-BB conjugates added. After 4 days, 15 μl (1 mg/ml) of MTT was added and after another 4 h, 150 μl of DMSO was added. After 16 hr, the optical density at 570 nm was determined using an ELISA reader.
Results
Hem-BB and Dol-BB conjugates bind with high affinity to cells containing BB2R
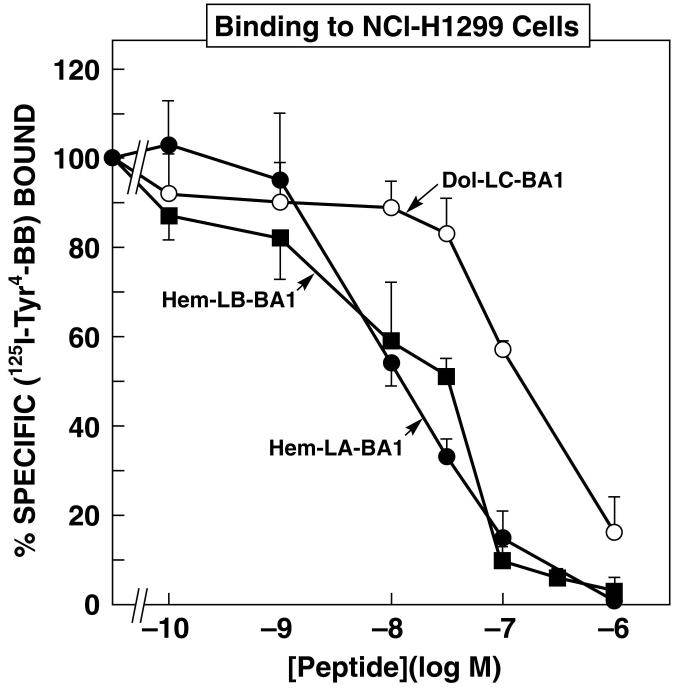
Figure 2 shows that specific (125I-Tyr4)BB binding to NCI-H1299 cells was inhibited in a dose-dependent manner by Hem-BB and Dol-BB conjugates. Hem-LA-BA1, Hem-LB-BA1 and Dol-LC-BA1 half maximally inhibited specific (125I-Tyr4)BB binding with IC50 values of 15, 25 and 150 nM, respectively. These results indicate that when the C-terminal of Hem was coupled to the N-terminal of BA1, the resulting conjugate bound with high affinity to lung cancer cells using either linker LA or LB. Table I shows that specific (125I-Tyr4)BB binding to NCI-H1299 cells was inhibited with high affinity by BA2, Dol-LC-BA1, Dols-LD-BA1 and Dols-LE-BA1 with IC50 values of 6, 150, 20 and 15 nM, respectively. These results indicate that when the C-terminal of Dol or Dols was linked to the N-terminal of BA1, the resulting conjugates bound with high affinity to cells containing BB2R.
Figure 2.
Binding to NCI-H1299 cells. The ability of varying concentrations of Hem-LA-BA1 (●), Hem-LB-BA1 (∎) and Dol-LC-BA1 (○) to inhibit specific (125I-Tyr4)BB binding to NCI-H1299 cells was investigated. The mean value ± S.D. of 4 determinations is indicated. This experiment is representative of 3 others.
Table I.
Binding to cells containing BB receptors
| NCI-H1299 | Balb/3T3 with BB2R | Balb/3T3 with BB1R | Balb/3T3 with BRS-3 | |
|---|---|---|---|---|
| Peptide | IC50, nM | |||
| BA2 | 6 ± 1 | 0.6 ± 0.1 | 12 ± 1 | 14 ± 2 |
| Dol-LC-BA1 | 150 ± 18 | 21 ± 2 | 270 ± 30 | 370 ± 60 |
| Dols-LD-BA1 | 20 ± 2 | 4 ± 1 | 190 ± 30 | 380 ± 40 |
| Dols-LE-BA1 | 15 ± 1 | 18 ± 2 | 1120 ± 90 | 1420 ± 130 |
| Hem-LA-BA1 | 15 ± 2 | 8 ± 1 | 130 ± 10 | 540 ± 50 |
| Hem-LB-BA1 | 25 ± 3 | 5 ± 1 | 69 ± 9 | 160 ± 30 |
The mean value ± S.D. of 3 determinations each repeated in quadruplicate is indicated. (125I-Tyr4)BB was bound to NCI-H1299 cells for 20 min at 37°C. 125I-BA2 was bound to Balb/3T3 cells for 60 min at 25°C. The structures of the peptides are shown below:
BA1 DPhe-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2
BA2 DTyr-Gln-Trp-Ala-Val-βAla-His-Phe-Nle-NH2
The ability of Hem-BB and Dol-BB conjugates to bind to Balb 3T3 cells stably transfected with human BB receptors was investigated. Table I shows that using Balb 3T3 cells transfected with BB2R, Hem-LA-BA1, Hem-LB-BA1 and Dol-LC-BA1 inhibited specific 125I-BA2 binding with IC50 values of 8, 5 and 21 nM, respectively. These results indicate that Hem-BB and Dol-BB conjugates bound with similar affinity to Balb 3T3 cells transfected with BB2R and human lung cancer cells. Hem-LA-BA1, Hem-LB-BA1 and Dol-LC-BA1 inhibited specific 125I-BA2 binding to Balb 3T3 cells transfected with human BB1R with IC50 values of 130, 69 and 270 nM, respectively. Hem-LA-BA1, Hem-LB-BA1 and Dol-LC-BA1 inhibited specific 125I-BA2 binding to Balb/3T3 cells transfected with human BRS-3 with IC50 values of 540, 160 and 370 nM, respectively. These results indicate that Hem-BB and Dol-BB conjugates bind with 1-2 orders of magnitude lower affinity the Balb 3T3 cells transfected with BB1R or BRS-3 relative to BB2R.
Hem-BB and Dol-BB conjugates elevate cytosolic Ca2+
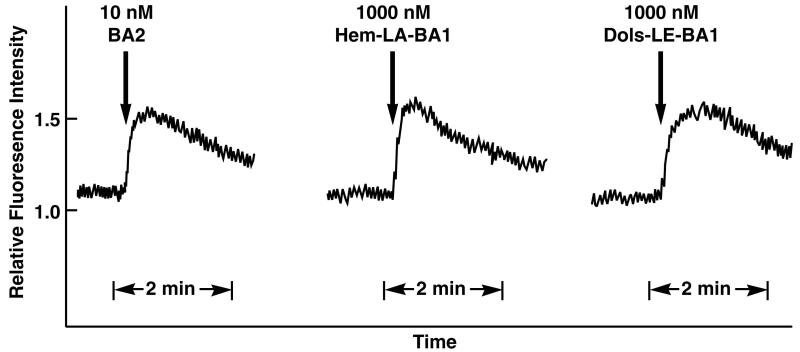
The effects of Hem-BB and Dol-BB conjugates were investigated on Fura-2/AM loaded NCI-H1299 or Balb 3T3 cells containing BB2R. Figure 3 shows that BA2, Hem-LA-BA1 and Dols-LE-BA1 caused elevated cytosolic Ca2+using BB2R containing cells. Using 1000 nM Hem-LA1-BA1 or Dols-LE-BAI, the fluorescence intensity strongly increased within seconds after addition to cells containing BB2R. The response was maximal after 0.2 min then slowly declined over a 2 min period. Similarly, 10 nM BA2 caused a strong Ca2+ response in 3T3 cells containing BB2R or NCI-H1299 cells as did 1000 nM Hem-LB-BA1, 1000 nM Dol-LC-BA1 and 1000 nM Dols-LD-BA1 (data not shown). The results indicate that Hem-BB and Dol-BB conjugates function as BB2R agonists.
Figure 3.
Cytosolic calcium. The abililty of (left) 10 nM BA2, (middle) 1000 nM Hem-LA-BA1 or (right) 1000 nM Dols-LE-BA1 to elevate Ca2+ in Fura-2AM loaded Balb 3T3 cells containing BB2-R is indicated. This experiment is representative of 2 others.
Hem-BB but not Dol-BB conjugates are cytotoxic for NCI-H1299 cells
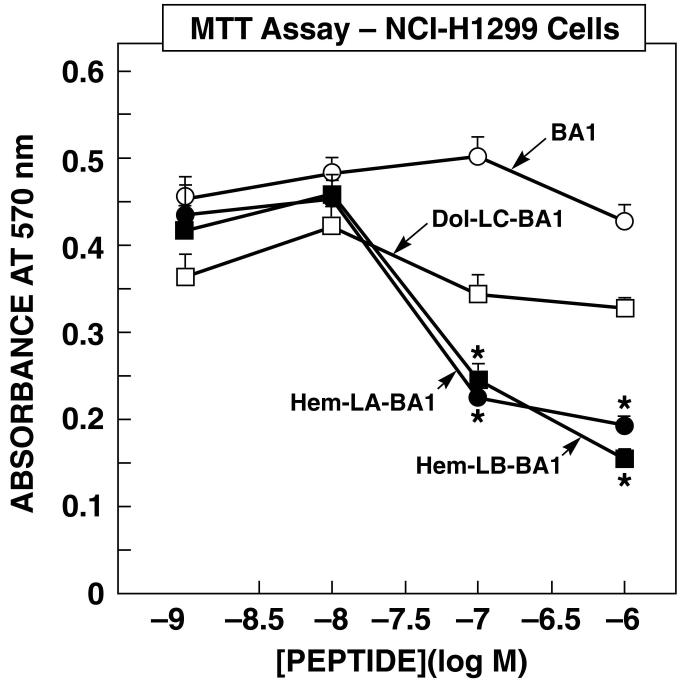
The ability of Hem-BB and Dol-BB conjugates to alter the proliferation of lung cancer cells was investigated. Figure 4 shows that Hem-BB but not Dol-BB conjugates or BA1 inhibited the proliferation of NCI-H1299 cells in a concentration dependent manner. Hem-LA-BA1 or Hem-LB-BA1 did not alter NCI-H1299 proliferation at 1 or 10 nM but significantly inhibited proliferation at 100 or 1000 nM. In contrast, Dol-LC-BA1 or BA1 did not significantly alter NCI-H1299 proliferation at any dose tested. Similarly, Dols-LD-BA1 or Dols-LE-BA1 had little effect on NCI-H1299 proliferation (data not shown). These results suggest that Hem-BB conjugates but not Dol-BB conjugates are cytotoxic for NCI-H1299 cells.
Figure 4.
MTT assay. The ability of varying concentrations of Hem-LA-BA1 (●), Hem-LB-BA1 (∎), Dol-LC-BA1 (□) and BA1 (○) to inhibit NCI-H1299 growth was investigated. The mean value ± S.D. of 8 determinations is indicated; p < 0.05, * using Student’s t-test. This experiment is representative of 3 others.
Discussion
BB receptors are present in numerous human cancers (Reubi et al., 2002). Using invitro autoradiographic techniques, the BB2R is present in 100% of prostate cancers, 72% of breast cancers and 33% of SCLC. The BB1R is present in 46% of intestinal carcinoids. BRS-3 is present in 35% of bronchial carcinoids. In addition, BB1R and BB2R mRNA was detected in 100% and 64% respectively of the NSCLC cell lines examined (Siegfried et al., 1999). Agonists, but not antagonists, for each of the three BB receptor subclasses, are rapidly internalized (Slice et al., 1998). Radiolabeled synthetic analogues of BB-related peptides demonstrate these can be used to target tumors/tissues containing BB receptors and these analogues are internalized in high amounts by these tumors (Hoffman et al., 2003). Recently, an 111In derivative of BB was utilized to image prostate cancer tumors (deVisser et al., 2007). It remains to be determined if BB-like peptides can be utilized for early detection and/or treatment of cancer.
Peptide hormones which are coupled to cytotoxic agents have been shown to be effective at delivering targeted drugs (Breeman et al., 2007). We have shown that BB-CPT conjugates are cytotoxic for lung cancer cells invitro and invivo (Moody et al., 2006). CPT can be conjugated with the universal BB agonist BA2 with retention of high affinity binding and biological activity (Moody et al., 2004). The BB-CPT conjugate bound with higher affinity to BB1R, BB2R or BRS-3 containing cells with higher affinity than did BA2, possibly because CPT increased hydrophobic interactions with BB receptors. The BB-CPT conjugate was internalized by and cytotoxic for cells containing BB1 or BB2 receptors as well as BRS-3. The CPT-BB conjugate inhibited NCI-H1299 xenograft growth in nude mice (Moody et al., 2006). Furthermore the BB-CPT conjugate inhibited the growth of 13 additional human cancer cell lines tested as well as 3 tumors. The results demonstrated that BB-CPT conjugates can be utilized in tumors which have BB2R, BB1R and/or BRS-3 and that BA2 is an excellent ligand to couple cytotoxic agents to allow BB receptor targeted delivery.
In this communication, BA1 was coupled to the marine natural products Hem or Dol. Hem-LA-BA1 bound with high affinity to cells containing BB2 receptors (IC50 = 8 nM) and lower affinity to cells containing BB1R or BRS-3 (IC50 = 130 and 540 nM, respectively). Hem-LB-BA1, which has a neutral linker of Ala-Leu-Ala-Leu-Ala, bound with similar affinity to NCI-H1299 cells as did Hem-LA-BA1, which has an acidic linker of Ala-Leu-Ala-Leu-Glu-Gly-Glu-Gly-Glu-Gly. These results suggest Hem-BB conjugates bind with high affinity to cells containing BB2R regardless of the linker used. In addition Dols-LD-BA1 bound with high affinity to cells containing BB2R (IC50 = 4 nM) and lower affinity to cells containing BB1R or BRS-3 (IC50 = 190 and 380 nM, respectively). Hem is a tripeptide composed of trimethyltryptophan, tert-leucine and N-methylhomovinylvaline (Fig. 1). Dol is a hexapeptide which has 5 of the same 6 amino acids as does Dol-15. Dol and Dol-15 have the same N-terminal dolavaline which is essential for interaction with cancer cell tubulin and the resulting cytotoxicity (Zask et al., 2005). Dol-15 interacts with tubulin binding sites similar to those of the traditional chemotherapeutic agents vincristine, vinblastin or vinorelbine (Bai et al., 1999).
The Hem-BB and Dol-BB conjugates functioned as agonists. They increased the cytosolic Ca2+ in cells containing BB2R. Previously, we showed that BB-CPT conjugates functioned as agonists in that they increased phosphatidylinositol turnover in cells containing BB2R, BB1R or BRS-3 (Moody et al., 2004). Also, the BB-CPT conjugate, which contains a Tyr, was iodinated and found to be internalized by NCI-H1299 cells. Because the Hem-BB or Dol-BB conjugates lack Tyr, it was not possible to iodinate them and determine if they were internalized. Previously, we found that all BB agonists examined which bound with high affinity to the BB2R, were internalized at 37°C but not 4°C (Benya et al., 1995; Mantey et al., 1997; Moody et al., 2004; Moody et al., 2006).
The Hem-BB but not Dol-BB conjugates were cytotoxic for NCI-H1299 cells. The IC50 values for Hem-LA-BA1 and Hem-LB-BA1 were 50 and 60 nM, respectively. In contrast, the Dol-BB conjugates or BA1 had no effect on NCI-H1299 cellular toxicity. It is possible that the Dol used, which had a C-terminal Phe, is not as biologically active as is Dol-15, which has a C-terminal 2-hydroxy-isovaline-dola-pyrrolidone. Dol-15 causes apoptosis of A549 lung cancer cells by causing a G2-M cell cycle arrest (Catassi et al., 2006). Dol-15 caused BAD dissociation from 14-3-3 followed by association with BCL-XL, cytochrome c release, caspase-3 activation and cleavage of vimentin. The Hem-BB conjugates (Hem interacts with tubulin) are more cytotoxic for NCI-H1299 cells than BB-CPT conjugates (CPT inhibits topoisomerase 1) which had an IC50 of 190 nM in the MTT assay.
The Hem and Dol conjugates are linked to BA1 through amide bonds. After undergoing receptor-mediated endocytosis, Hem can be released intracellularly from BA1 by endopeptidase metabolism of the linker. In contrast, the BB-CPT conjugates are linked via a carbamate bond and are metabolized by P450 enzymes. These results suggest that the cell surface BB2R receptor can serve as a molecular target to deliver marine toxins into cancer cells. In contrast, normal cells which lack the BB2R should not be affected by the Hem-BB conjugates. Because there is no receptor for Hem or Dol, the marine toxins may diffuse into plasma membranes and kill both normal and cancer cells, leading to toxic side effects. The Hem-BB conjugates will be cytotoxic for cancer cells enriched in BBR, endopeptidases and tubulin, and should have little toxicity for cells lacking BB receptors. Hem-LA-BA1 will have greater selectivity for cancer cells containing BB2R than will Hem-LB-BA1. While it will be easier to synthesize Hem-LB-BA1 than Hem-LA-BA1 due to the smaller linker size, Hem-LB-BA1 will likely be cytotoxic for cells containing BB1R, BB2R and/or BRS-3. It remains to be determined if Hem will be preferentially released by cancer cell enzymes using Hem-LA-BA1 or Hem-LB-BA1.
Conclusions
Hem-LA-BA1 binds with high affinity cells containing BB2R. Because the Hem-LA-BA1 functions as an agonist, it may be internalized by lung cancer cells. It remains to be determined if Hem-LA-BA1 may be a useful conjugate for treatment of cancer patients whose tumors are enriched in BB2R.
Acknowledgments
This manuscript is dedicated to the memory of the late Christopher Michejda. This research was partially supported by intramural research funds of the NIDDK and NCI, of NIH.
References
- Bai R, Durso NR, Sackett DL, Hamel E. Interactions of the sponge derived antimitotic tripeptide hemiasterlin with tubulin: Comparison with dolastatin 10 and cryptophycin 1. Biochemistry. 1999;38(43):14302–14310. doi: 10.1021/bi991323e. [DOI] [PubMed] [Google Scholar]
- Battey JF, Way J, Corjay MH, Shapira H, Kusano K, Harkins R, Wu JM, Slattery T, Mann E, Feldman R. Molecular cloning of the bombesin/GRP receptor from Swiss 3T3 cells. Proceedings of the National Academy of Sciences of the United States. 1991;88(2):395–399. doi: 10.1073/pnas.88.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benya RV, Kusui T, Battey JF, Jensen RT. Chronic desensitization and down-regulation of the gastrin-releasing peptide receptor are mediated by a protein kinase C-dependent mechanism. Journal of Biological Chemistry. 1995;270(14):3346–3352. doi: 10.1074/jbc.270.7.3346. [DOI] [PubMed] [Google Scholar]
- Breeman WA, Kwekkeboom DJ, de Blois E, de Jone M, Visser TJ, Krenning EP. Radiolabelled regulatory peptides for imaging and therapy. Anti-cancer Agents in Medicinal Chemistry. 2007;7(3):345–357. doi: 10.2174/187152007780618171. [DOI] [PubMed] [Google Scholar]
- Buchholz S, Keller G, Schally AV, Halmos G, Hohla F, Heinrich E, Koester F, Baker B, Engel JB. Therapy of ovarian cancers with targeted cytotoxic analogs of bombesin, somatostatin, and luteinizing hormone-releasing hormone and their combinations. Proceedings of the National Academy of Sciences of the United States. 2006;103(27):10403–10407. doi: 10.1073/pnas.0602971103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catassi A, Cesario A, Arzani D, Menichini P, Alama A, Bruzzo C, Imperatori A, Rotolo N, Granone P, Russo P. Characterization of apoptosis induced by marine natural products in non small cell lung cancer A549 cells. Cellular and Molecular Life Sciences. 2006;63(1920):2377–2386. doi: 10.1007/s00018-006-6264-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochemical Pharmacology. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Cuttitta F, Carney DN, Mulshine J, Moody TW, Fedorko J, Fischler A, Minna JD. Bombesin-like peptides can function as autocrine growth factors in human small cell lung cancer. Nature. 1985;316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- deGroot FM, Damen EW, Scheeren HW. Anticancer prodrugs for application in monotherapy: Targeting hypoxia, tumor-associated enzymes, and receptors. Current Medicinal Chemistry. 2001;8(9):1093–1122. doi: 10.2174/0929867013372634. [DOI] [PubMed] [Google Scholar]
- deVisser M, Bernard HF, Erion JL, Schmidt MA, Srinivasan A, Waser B, Reubi JC, Krenning EP, de Jong M. Novel (111)In-labeled bombesin analogues for molecular imaging of prostate tumours. European Journal of Nuclear Medicine and Molecular Imaging. 2007;34(8):1228–1238. doi: 10.1007/s00259-006-0356-3. [DOI] [PubMed] [Google Scholar]
- Fathi Z, Corjay MH, Shapira H, Wada E, Benya R, Jensen R, Viallet J, Sausville EA, Battey JH. BRS-3: A novel bombesin receptor subtype selectively expressed in testis and lung carcinoma cells. Journal of Biological Chemistry. 1993;268(8):5979–5984. [PubMed] [Google Scholar]
- Hoffman TJ, Gali H, Smith CJ, Sieckman GL, Hayes CL, Owen NK, Volkert WA. Novel series of 111In-labeled bombesin analogs as potential radiopharmaceuticals for specific targeting of gastrin-releasing peptide receptors expressed on human prostate cancer cells. Journal of Nuclear Medicine. 2003;44(5):823–831. [PubMed] [Google Scholar]
- Jensen RT, Moody TW. Bombesin-related peptides and neurotensin: Effects on cancer growth/proliferation and cellular signaling in cancer. In: Kastin AJ, editor. Handbook of biologically active peptides. Elsevier; Amsterdam: 2006. pp. 429–434. [Google Scholar]
- Kobayashi M, Natsume T, Tamaoki S, Watanabe J, Asano H, Mikami T, Miyasaka K, Miyazaki K, Gondo M, Sakakibara K, Tsukagoshi S. Antitumor activity of TZT-1027, a novel dolastatin 10 derivative. Japanese Journal of Cancer Research. 1997;88(2):316–327. doi: 10.1111/j.1349-7006.1997.tb00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisnamurthy G, Cheng W, Mei-Chur L, Aulabaugh A, Razinkov V, Ding W, Loganzo F, Zaks A, Ellestad G. Biophysical characterization of the interactions of HTI-286 with tubulin heterodimer and microtubules. Biochemistry. 2003;42(46):13484–13495. doi: 10.1021/bi035530x. [DOI] [PubMed] [Google Scholar]
- Mantey SA, Weber HC, Sainz E, Akeson M, Ryan RR, Pradhan T, Searles RP, Spindel ER, Battey JF, Coy DH, Jensen RT. Discovery of a high affinity radioligand for the human orphan receptor, bombesin receptor subtype 3, which demonstrates it has a unique pharmacology compared to other mammalian bombesin receptors. Journal of Biological Chemistry. 1997;272(41):26062–26071. doi: 10.1074/jbc.272.41.26062. [DOI] [PubMed] [Google Scholar]
- Moody TW, Mantey SA, Pradhan TK, Schumann R, Nakagawa T, Martinez A, Fusilier J, Coy DH, Jensen RT. Development of high affinity camptothecin-bombesin conjugates that have targeted cytotoxicity for bombesin receptor-containing tumor cells. Journal of Biological Chemistry. 2004;279(22):23580–23589. doi: 10.1074/jbc.M401938200. [DOI] [PubMed] [Google Scholar]
- Moody TW, Murphy A, Mahmoud S, Fiskum G. Bombesin-like peptides elevate cytosolic calcium in small cell lung cancer cells. Biochemical and Biophysical Research Communications. 1987;147(1):189–195. doi: 10.1016/s0006-291x(87)80105-5. [DOI] [PubMed] [Google Scholar]
- Moody TW, Pert CB, Gazdar AF, Carney DN, Minna JD. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Moody TW, Sun LC, Mantey SA, Pradhan T, Mackey LV, Gonazlez N, Fuselier JA, Coy DH, Jensen RT. In vitro and in vivo antitumor effects of cytotoxic camptothecin-bombesin conjugates are mediated by specific interaction with cellular bombesin receptors. Journal of Pharmacology and Experimental Therapeutics. 2006;318(3):1265–1272. doi: 10.1124/jpet.106.104141. [DOI] [PubMed] [Google Scholar]
- Nagy A, Schally AV. Targeting cytotoxic conjugates of somatostatin, luteinizing hormone-releasing hormone and bombesin to cancers expressing their receptors: a “smarter” chemotherapy. Current Pharmaceutical Design. 2005;11(9):1167–1180. doi: 10.2174/1381612053507594. [DOI] [PubMed] [Google Scholar]
- Patterson LH, McKeown SR, Robson T, Gallagher R, Raleigh SM, Orr S. Antitumor prodrug development using cytochrome P450 (CYP) mediated activation. Anti-cancer Drug Design. 1999;14(6):473–474. [PubMed] [Google Scholar]
- Pettit GR, Kamano Y, Dufresne C, Cerny RL, Herald CL, Schmidt JM. Isolation and structure of the cytostatic linear depsipeptide dolastatin 15. Journal of Organic Chemistry. 1989;54(26):6005–6006. [Google Scholar]
- Pitot HC, McElroy EA, Reid JM, Windebank AJ, Sloan JA, Erlichman C, Baniewski PG, Walker DL, Rubin J, Goldberg RM, Adjei AA, Ames MM. Phase I trial of dolastatin-10 (NSC 376128) in patients with advanced solid tumors. Clinical Cancer Research. 1999;5(3):525–531. [PubMed] [Google Scholar]
- Pradhan TK, Katsuno T, Taylor JE, Kim SH, Ryan RR, Mantey SA, Donohue PJ, Weber HC, Sainz E, Battey JF, Coy DH, Jensen RT. Identification of a unique ligand which has high affinity for all four bombesin receptor subtypes. European Journal of Pharmacology. 1998;343(23):275–287. doi: 10.1016/s0014-2999(97)01527-6. [DOI] [PubMed] [Google Scholar]
- Rawat DS, Joshi MC, Joshi P, Atheaya H. Marine peptides and related compounds in clinical trial. Anti-Cancer Agents in Medicinal Chemistry. 2006;6(1):33–40. doi: 10.2174/187152006774755519. [DOI] [PubMed] [Google Scholar]
- Reubi JC, Wenger S, Schumuckli-Maurer J, Schaer JC, Gugger M. Bombesin receptor subtypes in human cancers: Detection with the universal radioligand (125)I-D-TYR(6), beta-ALA(11), PHE(13), NLE(14) bombesin(6-14) Clinical Cancer Research. 2002;8(4):1139–1146. [PubMed] [Google Scholar]
- Sato M, Sagawa M, Nakazato T, Ikeda Y, Kizaki M. A natural peptide, dolastatin 15, induces G2/M cell cycle arrest and apoptosis of human multiple myeloma cells. International Journal of Oncology. 2007;30(6):1453–1459. [PubMed] [Google Scholar]
- Sekido Y, Fong KM, Minna JD. Molecular biology of lung cancer. In: DeVita VT, Jelman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Lippincott Williams and Wilkins; Philadelphia: 2005. pp. 745–752. [Google Scholar]
- Siegfried JM, Krrishnamachary N, Gaiter A, Gublish C, Hunt JD, Shriver SP. Evidence for autocrine actions of neuromedin B and gastrin-releasing peptide in non-small cell lung cancer. Pulmonary Pharmacological Therapy. 1999;12(5):291–302. doi: 10.1006/pupt.1999.0210. [DOI] [PubMed] [Google Scholar]
- Slice LW, Yee HF, Jr., Walsh JH. Visualization of internalization and recycling of the gastrin releasing peptide receptor-green fluorescent protein chimera expressed in epithelial cells. Receptors and Channels. 1998;6(3):201–12. [PubMed] [Google Scholar]
- Spindel ET, Giladi E, Brehm TP, Goodman RH, Segerson TP. Cloning and functional charactrerization of a cDNA encoding the murine fibroblast bombesin/GRP receptor. Molecular Endocrinology. 1990;4(12):1956–1963. doi: 10.1210/mend-4-12-1956. [DOI] [PubMed] [Google Scholar]
- Talpir R, Benayahu Y, Kashman Y, Pannell L, Schleyer M. Hemiasterlin and geodiamolide TA: Two new cytotoxic peptides from marine sponge hemiasterellminor (Kirkpatric) Tetrahedron Letters. 1994;35(25):4453–4456. [Google Scholar]
- Wada E, Way J, Shapira H, Kusano K, Lebacq-Verheyden AM, Coy D, Jensen R, Battey JF. cDNA cloning, characterization and brain region specific expression of a neuromedin-B preferring receptor. Neuron. 1991;6(3):421–430. doi: 10.1016/0896-6273(91)90250-4. [DOI] [PubMed] [Google Scholar]
- Wood SM, Wood JR, Ghatei MA, Lee YC, O’Shaughnessy D, Bloom SR. Bombesin, somatostatin and neurotensin-like immunoreactivity in bronchial carcinoma. Journal of Clinical Endocrinology and Metabolism. 1981;53(6):1310–1312. doi: 10.1210/jcem-53-6-1310. [DOI] [PubMed] [Google Scholar]
- Zask A, Kaplan J, Musto S, Loganzo F. Hybrids of hemiasterlin analogue taltobulin and the dolastatins are potent antimicrotubule agents. Journal of American Chemical Society. 2005;127(50):17667–17671. doi: 10.1021/ja053663v. [DOI] [PubMed] [Google Scholar]