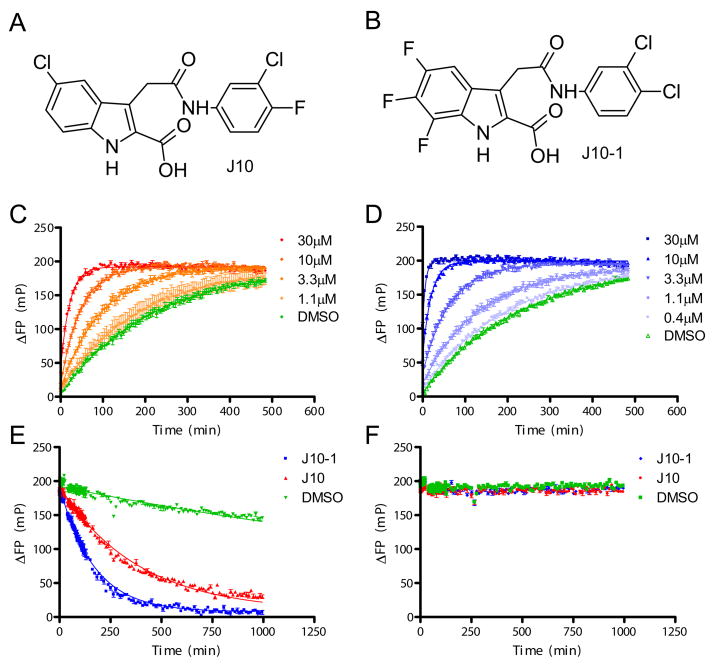
Figure 1. Properties of the small molecule catalysts of peptide exchange.
A & B. Chemical structures of the original lead compound J10 and the most potent analog prepared, J10-1. C & D. Acceleration of peptide binding to DR15 by J10 (C) or J10-1 (D) measured using a fluorescence polarization (FP) assay. Increasing concentrations of J10 (1.1–30 μM) or J10-1 (0.4–30 μM) were incubated with 150 nM DR15/CLIP complex and 30 nM MBP-488 in 50 mM sodium citrate buffer, 150 mM NaCl, pH 5.2. ΔFP values (in millipolarization units, mP) were obtained by subtraction of FP values from wells containing only MBP-488. The binding curve of MBP-488 to DR15 in the absence of a small molecule is shown in green. E & F. J10 and J10-1 catalyze peptide dissociation from DR15. Dissociation of the MBP-488 peptide from the DR15/MBP-488 complex (100 nM) was measured over time by FP in the presence of 100 μM J10 (red line), 100 μM J10-1 (blue line) or no small molecule (DMSO control, green line). MBP-488 dissociation only occurred in the presence of 50 μM unlabeled pMBP competitor peptide (E), but not in its absence (F), indicative of rapid rebinding of MBP-488 to DR15.