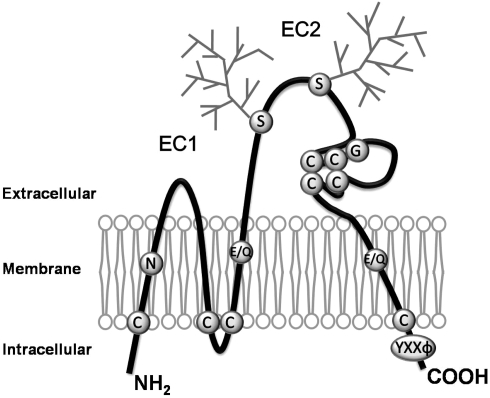
Figure 1.
Schematic drawing of the structure of tetraspanins. Tetraspanins are composed of four transmembrane, an intracellular N- and C-termini, and two extracellular (one shorter, EC1, and one longer, EC2) domains. The conserved motifs or residues featured by tetraspanins are denoted. Most tetraspanins contain four or six cysteine residues in EC2, and two of those are in a highly conserved ‘CCG’ motif. Most of tetraspanins have glycosylation sites in EC2 as indicated as the squares, while CD9 is glycosylated in EC1. In each tetraspanin, there are several cysteine residues proximal to the interface of the inner leaflet and cytosol, which are the sites for palmitoylation and characteristic strong polar residues in the transmembrane domains. Tetraspanins also contains ‘YXXΦ’ sorting motif in the C-terminal cytoplasmic domain. C, Cysteine; E, Glutamic acid; G, Glycine; Q, Glutamine; N, Aspargine; S, Serine; Y, Tyrosine; X, any aminoacid; Φ, Hydrophobic aminoacid.