Abstract
Aims
In this study, we wished to determine whether angiopoietin-1 (Ang1) modified the permeability coefficients of non-inflamed, intact continuous, and fenestrated microvessels in vivo and to elucidate the underlying cellular mechanisms.
Methods and results
Permeability coefficients were measured using the Landis–Michel technique (in frog and rat mesenteric microvessels) and an oncopressive permeability technique (in glomeruli). Ang1 decreased water permeability (LP: hydraulic conductivity) in continuous and fenestrated microvessels and increased the retention of albumin (σ: reflection coefficient) in continuous microvessels. Endothelial glycocalyx is common to these anatomically distinct microvascular beds, and contributes to the magnitude of both LP and σ. Ang1 treatment increased the depth of endothelial glycocalyx in intact microvessels and increased the content of glycosaminoglycan of cultured microvascular endothelial cell supernatant. Ang1 also prevented the pronase-induced increase in LP (attributable to selective removal of endothelial glycocalyx by pronase) by restoration of glycocalyx at the endothelial cell surface. The reduction in permeability was inhibited by a cell transport inhibitor, Brefeldin.
Conclusion
Ang1 modifies basal microvessel permeability coefficients, in keeping with previous reports demonstrating reduced solute flux in inflamed vessels. Anatomical, biochemical, and physiological evidence indicates that modification of endothelial glycocalyx is a novel mechanism of action of Ang1 that contributes to these effects.
KEYWORDS: Permeability, Angiopoietin-1, Glycocalyx, Microvessel, Glomerulus
1. Introduction
Ang1 is a 70-kDa glycoprotein that shares homology with a number of other angiopoietins and angiopoietin-like molecules.1 Their expression and activity have been demonstrated across a range of vertebrate families encompassing mammals2,3 and amphibians.4 Ang1 is a ligand for the tyrosine kinase receptor Tie2, expressed by endothelial cells throughout the vasculature,1 and a small number of other cell types.5 Ang1 is constitutively expressed and its receptor, Tie2, is constitutively phosphorylated in many adult tissues.3 Endothelial cell responses to Ang1 in vitro include chemotaxis, prevention of apoptosis, sprouting, and tube formation.1 Activities in vivo include reduction of leukocyte adhesion and other markers of inflammation,6 and tissue-specific and time-specific alterations in angiogenesis.7,8
The primary function of the microvasculature is to regulate exchange of substances between plasma and abluminal compartments (e.g. interstitium, or urinary space in the case of glomeruli). The mass of solute and solvent crossing microvascular walls is determined by
the forces acting across microvessel walls that drive solute/solvent flux (net hydrostatic and oncotic pressure gradients, concentration gradients),
haemodynamic forces (plasma flow rate, area available for exchange), and
the permeability of microvessel walls.
Microvascular permeability [point (iii)] is described by three permeability coefficients: hydraulic conductivity (LP: the ease with which water crosses microvessel walls), reflection coefficient (σ: the fraction of a solute that cannot be dragged across microvessel walls with the convective flux of solvent), and the diffusive solute permeability coefficient (PS: the degree to which microvessel walls hinder the diffusive movement of solute from lumen to abluminal compartment).
All previous studies that have attempted to investigate the effect of Ang1 on microvascular permeability in intact vessels have measured solute (or solvent) flux (determined by all three factors listed above), rather than measuring microvascular permeability (i.e. permeability coefficients) per se. The Miles Assay,9 leak of fluorescent microspheres10 and paw oedema11 are all measures of the composite parameters of solute/solvent flux, rather than specific measures of the contribution of microvessel permeability coefficients to solute flux. Because Ang1 affects arteriolar resistance,12 changes in hydrostatic pressure gradients, area available for exchange, plasma flow rate, and consequently concentration gradients are all likely to occur in response to Ang1.13 These studies are limited in their ability to reveal the mechanisms by which Ang1 alters total solute flux in intact vessels in vivo, particularly under the baseline conditions under which the Ang1–Tie2 axis is active.3 Ang1 would be predicted to alter solute flux by modulating the contribution of the endothelium to microvascular permeability, given the effects of this ligand on endothelial cell biology, and techniques that can discriminate changes in permeability coefficients from changes in haemodynamic forces and driving forces are necessary to demonstrate the contribution of altered permeability coefficients.
Evidence that Ang1 modifies permeability coefficients has been provided by in vitro studies, in which application of Ang1 to endothelial cell monolayers reduces both water and albumin flux,14,15 predominantly via alteration of the activity and expression of molecules involved in the integrity of inter-endothelial cell junctions, such as VE-cadherin, PECAM-1, and β-catenin.14 It is critical to note, however, that the permeability coefficients of endothelial cell monolayers are both quantitatively and qualitatively different from those of intact microvessels in vivo: indeed, the permeability coefficients of endothelial cell monolayers are comparable to those of inflamed microvessels.16 Ang1 has a multiplicity of anti-inflammatory actions, and all of the aforementioned studies of solute–solvent flux in vivo have examined the effects of Ang1 following an inflammatory stimulus. Ang1, however, is typically expressed under baseline (i.e. non-inflamed) conditions, and the effects of Ang1 on microvascular permeability and/or solute–solvent flux have not been examined under these baseline, non-inflamed conditions.
We therefore sought to examine whether Ang1 modifies microvascular permeability coefficients under conditions in which all other determinants of transvascular solute and solvent movement are known and controlled, and to determine these influences in the resting state, i.e. without prior induction of inflammation.
We also sought to compare the effects of Ang1 on the hydraulic conductivity of microvessels with continuous and fenestrated endothelia. Paracellular routes (i.e. through the inter-endothelial cleft) dominate fluid flux in continuous capillaries,17 but transcellular routes (i.e. through the fenestrations) dominate fluid flux in fenestrated capillaries.18 If Ang1 modifies permeability coefficients in intact microvessels via modification of inter-endothelial cleft molecules, then changes in continuous microvessel water permeability (LP), but not fenestrated microvessel LP, would be expected in response to Ang1.
2. Methods
2.1. Animal preparation
Experiments on adult frogs (Rana temporaria) and rats (Wistar) were performed in accordance with both the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and UK Home Office legislation. Mesenteric permeability experiments were performed as described by Bates and coworkers,19 and glomerular permeability experiments as reported by Salmon et al.20 (see Supplementary material online).
2.2. Systemic microvessel permeability coefficient measurement
In view of the difficulties in separating permeability coefficients using more widely used methodologies, we used the Landis–Michel micro-occlusion technique, as we have previously described in intact microvessels in vivo19 (see Supplementary material online for details). This technique allows the precise measurement of permeability characteristics, as the intravascular forces that determine transvascular fluid and solute flux (hydrostatic and oncotic pressures) are controlled. Individual microvessels (16–37 µm; capillaries and post-capillary venules) of known surface area in the mesentery were cannulated with a bevelled micropipette, while the mesentery was superfused with Ringer's solution. The vessels were then perfused with bovine serum albumin (BSA) at a known concentration, and erythrocytes (as flow markers), at a defined pressure. To measure LP and σ, vessels were occluded with a fine glass rod. Fluid filtration rate per unit area was calculated from the rate of erythrocyte movement and the cross-sectional area of the vessel. LP (×10−7 cm s−1 cm H2O−1) was calculated from the slope of the relationship between filtration rate and applied pressure, and σ from the abscissal intercept.
Permeability coefficients were measured before and after microvessel perfusion with recombinant human Ang1 (R&D Systems; 200 ng mL−1) or vehicle (1× phosphate-buffered saline). In separate experiments on frog microvessels, the ability of Ang1 to inhibit the pronase-induced increase in LP that has previously been reported in the same system21 was assessed by measuring LP during pre-perfusion of the microvessel with either Ang1 or vehicle solution for 30 min, followed by LP measurement after brief and transient (<90 s) microvessel perfusion with 0.1 mg mL−1 pronase, following which the initial perfusate (Ang1 or BSA) was restored (please see Supplementary material online for details). LP was also measured in vessels that were only perfused with Ang1 following pronase exposure. To examine whether Ang1 inhibits the translocation of pre-formed glycocalyx constituents from intracellular stores, frog microvessels were co-perfused with Ang1 (200 ng mL−1) and the Golgi vesicle translocation inhibitor 100 µm Brefeldin-A.22
2.3. Electron microscopy
Frog microvessels were perfused initially with BSA-Ringer containing Ang1 or vehicle, with or without subsequent pronase exposure, as for permeability studies. Thereafter, perfusion with albumin-free Ringer solution for 1 min, then Alcian Blue solution for 5 min, was instituted. Vessels were then fixed by flooding the mesentery with 4% ice-cold gluteraldehyde in cacodylate buffer. After fixation, the position of the microvessel in the mesentery was drawn; the mesentery was cut away from the animal, stored in cacodylate-buffered 4% gluteraldehyde at 4°C overnight, post-fixed in 1% osmium tetroxide, and embedded in araldite blocks using standard techniques. The perfused microvessel was identified in the block, and transverse ultrathin (100 nm) sections were prepared. The sections were stained with lead citrate and uranyl acetate before being viewed in a Phillips 100CS electron microscope. Images were prepared from between two and four vessels per treatment group (mean: 3.25 vessels).
Electron microscopy (EM) images of microvessels were analysed with Adobe Photoshop (R). A calibrated electronic grid was applied to EM images and (i) glycocalyx depth (luminal edge of plasmalemma to luminal edge of glycocalyx); (ii) glycocalyx separation from the plasmalemma (luminal edge of plasmalemma to the nearest detectable glycocalyx); and (iii) inter-endothelial cleft width (wide cleft regions only) were measured at regular 50 nm intervals. In addition, the number of tight junctions (electron dense, narrow, regions of inter-endothelial clefts) were recorded for each treatment group, and divided into the total cleft length for each treatment group, to reveal the mean cleft length per tight junction.
2.4. Glomerular permeability coefficient measurement
Glomeruli were isolated from rat kidneys using a standard sieving technique,20 and treated with Ang1 or vehicle for 60 min in a blinded fashion. Individual glomeruli were loaded onto the tip of an aspiration micropipette within a flow-controlled glass observation chamber, and glomerular profile recorded during exchange of surrounding solution from 10 to 80 mg mL−1 BSA. The consequent rate of glomerular volume reduction (which represents the rate of flux of fluid from glomerular capillary lumina across the glomerular filtration barrier: JV) was analysed off-line. Glomerular hydraulic conductivity–surface area product (LPA) was calculated from the quotient of rate of volume change and applied oncotic pressure:
| 1 |
2.5. Endothelial cell glycosaminoglycan assay in vitro
Adult dermal human microvascular endothelial cells (Cambrex) were grown to 60–70% confluency, serum-starved for 24 h and then exposed to Ang1 or vehicle for 48 h. Supernatant was then aspirated, centrifuged, incubated with papain (to eliminate interference from proteins and glycoproteins23,24) and added to an Alcian Blue solution. The linear relation between mass of glycosaminoglycan and the degree of reduction in 488 nm light absorption by Alcian Blue solution25 was used to quantify supernatant glycosaminoglycan content.
2.6. Statistics
Mesenteric microvessel LP values are not normally distributed:26 results are reported as median ± semi-interquartile range, and analysed with non-parametric statistics. All other results are reported as mean ± SEM, and compared with parametric statistics. For permeability coefficient experiments, numerical results and associated statistical evaluations are given in Table 1.
3. Results
3.1. Angiopoietin-1 reduces LP of vessels with continuous endothelium
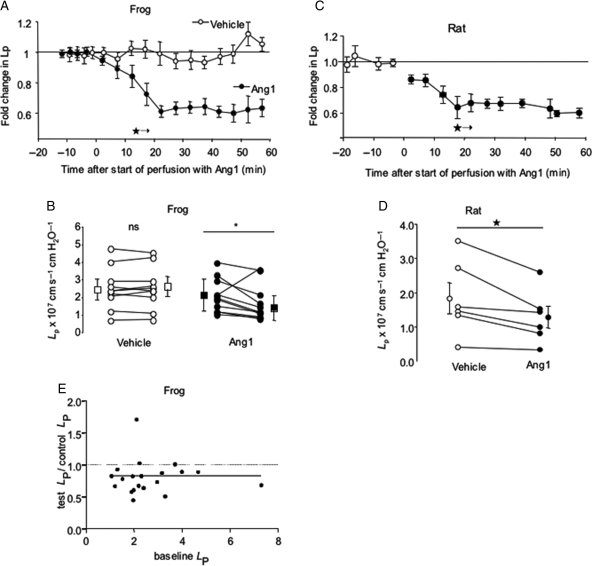
While LP was unaltered by BSA perfusion, luminal perfusion with 200 ng mL−1 Ang1 caused a significant reduction in LP of frog microvessels. The reduction in LP was complete after 20 min, and no further reductions in LP were observed during the following 40 min of perfusion with Ang1 (Figure 1A and B). Identical effects were observed in a mammalian system (rat mesenteric microvessels: Figure 1C and D). There was no relation between the magnitude of fall in LP and baseline LP (Figure 1E). The effect of Ang1 on LP was reversible, in that LP returned to baseline levels 30 min after removal of Ang1 from the perfusate (Figure 1, Supplementary material online).
Figure 1.
Angiopoietin-1 decreases continuous microvessel hydraulic conductivity (LP). LP was measured by cannulation and perfusion of individual mesenteric microvessels, and determination of transcapillary fluid flux under known hydrostatic capillary pressures. (A) Perfusion of 11 frog microvessels with 200 ng mL−1 Angiopoietin-1 (Ang1; closed symbols) caused a steady and sustained decrease in LP compared with 1% bovine serum albumin (BSA) alone (vehicle; open symbols) (*P < 0.05 vs. baseline, one-way analysis of variance for all subsequent timepoints). (B) Seventy minute-perfusion of frog mesenteric microvessels with BSA alone (vehicle; open circles; squares ± error bars represent median ± semi-IQR) caused no significant change in LP (nsP > 0.60, n = 10 pairs). In contrast, 70-min perfusion with BSA supplemented with 200 ng mL−1 Ang1 (filled circles) reduced LP in 10 of 11 vessels studied (*P < 0.05, Wilcoxon, n = 11 pairs). (C) As for frog vessels, Ang1 induced a significant reduction in rat mesenteric microvessel LP after 15 min exposure. This reduction again persisted for the remaining 45 min of perfusion (*P < 0.05 vs. baseline, one-way ANOVA for all subsequent timepoints). (D) Perfusion with 200 ng mL−1 rhAng1 caused a reduction in LP in all six rat mesenteric microvessels studied (*P < 0.05, Wilcoxon; squares ± error bars represent mean ± SEM). (E) There was no relation between baseline LP and the magnitude of fall in LP induced by Ang1 (Spearman r = 0.16; P > 0.4; n = 28 frog vessels).
3.2. Angiopoietin-1 increases σ of vessels with continuous endothelium
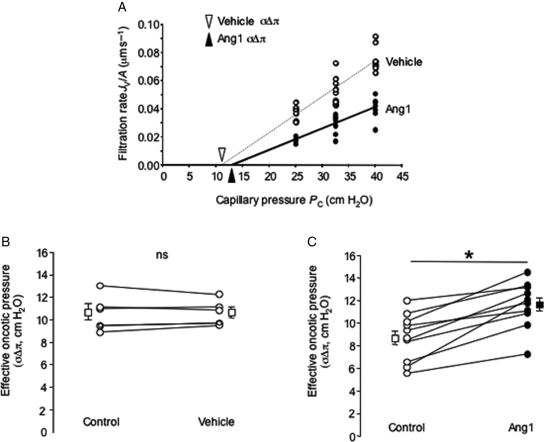
Measurement of filtration rate under increasing pressure in the vessel (Figure 2) was performed in 10 vessels. The slope of the relation between Jv and Pc (i.e. the hydraulic conductivity, LP) was (again) significantly reduced by Ang1. The abscissal intercept of the relation between Jv and Pc [i.e. the mean effective oncotic pressure (σΔπ) difference across the vessel wall] was significantly elevated by Ang1: this corresponds to an increase in albumin reflection coefficient (σalb, i.e. a decrease in macromolecular permeability) from 0.78 ± 0.03 to 0.91 ± 0.03 (n = 10 pairs; P < 0.001, paired t-test). This effect was observed in all the 10 vessels studied (Figure 2), and was observed even in vessels with the highest baseline values of σΔπ. As for LP, σ returned to baseline values 30 min after removal of Ang1 from the perfusate (Figure 1, Supplementary material online).
Figure 2.
Angiopoietin-1 increases continuous microvessel reflection coefficient (s). (A) A single frog mesenteric capillary perfused with 3% bovine serum albumin (BSA), submitted to a series of vessel occlusions made at a variety of luminal hydrostatic pressures (Pc) under baseline conditions (vehicle; open circles), and subsequently following 30-min perfusion with 200 ng mL−1 Angiopoietin-1 (Ang1; closed circles). LP, the slope of the relation between fluid flux rate per unit vessel area (Jv/A) and Pc was reduced by Ang1, and the x-axis intercept of this relation [triangle: effective oncotic pressure difference (sDp)] was increased. (B) σΔπ was unaltered by 30-min perfusion with vehicle (BSA alone: open circles; squares ± error bars represent mean ± SEM; n = 5 pairs; nsP > 0.9, paired t-test). (C) In contrast, 30-min treatment with Ang1 increased σΔπ (and hence σ) in all the 10 vessels studied (*P < 0.001, paired t-test) (squares ± error bars represent mean ± SEM).
3.3. Angiopoietin-1 reduces LPA of vessels with fenestrated endothelium
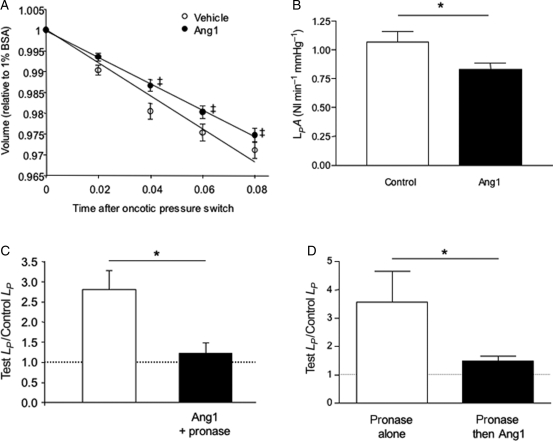
Treatment with 200 ng mL−1 Ang1 reduced the hydraulic conductivity of fenestrated rat glomerular capillaries to 76% of control, assuming no change in glomerular vascular area during the measurement (Figure 3). Ang1 was therefore able to reduce vascular permeability to water both in vessels with continuous and fenestrated endothelium.
Figure 3.
Angiopoietin-1 decreases glomerular water permeability and attenuates the pronase-induced increase in hydraulic conductivity (LP). Baseline glomerular volume was measured after 60 min exposure to 200 ng mL−1 Ang1 (n = 30) or vehicle (n = 31) in low oncotic pressure solution, and again immediately after exchange to high oncotic pressure solution (that induced fluid efflux from glomeruli). (A) The slope of the mean glomerular volume–time relation was significantly shallower for angiopoietin-1-treated glomeruli (*P < 0.05, one-way analysis of variance). (B) This reduction in the rate of fluid movement across the glomerular filtration barrier equates to a 24% reduction in glomerular LPA (*P < 0.05, unpaired t-test). (C) Ang1 pre-treatment prevented the pronase-induced increase in LP (*P < 0.05, unpaired t-test) in frog mesenteric microvessels. (D) LP remained elevated 30–45 min after initial exposure to pronase [LP 3.6 ± 1.1-fold higher than baseline (n = 4)]. LP values were restored towards baseline levels in pronase-exposed vessels subsequently perfused with 200 ng mL−1 rhAng1 (LP 1.5 ± 0.2-fold higher than baseline (n = 5; *P < 0.05 vs. pronase alone-treated vessels, Mann–Whitney U test).
We therefore investigated the possibility that Ang1 modified a structure that is present in both continuous and fenestrated microvessels, and contributes to hydraulic resistance and macromolecular sieving properties in both the vessel types: the endothelial glycocalyx.
3.4. Angiopoietin-1 prevents the pronase-induced increase in LP in vivo
Using the Landis–Michel technique, Adamson showed that brief perfusion of frog mesenteric microvessels with pronase caused both selective removal of the endothelial glycocalyx (but leaves the underlying endothelial cells intact) and a 2.5-fold increase in LP.21 We have used the same protocol in the same animal species to examine whether Ang1 modifies permeability coefficients by modifying endothelial glycocalyx.
Transient perfusion of vessels with pronase caused a 2.6 ± 0.4-fold increase in LP over baseline (n = 9). This pronase-induced increase in LP was attenuated to 1.2 ± 0.3-fold in vessels pre-perfused with Ang1 for 30 min (n = 6; P < 0.05, unpaired t-test; Figure 3C). Perfusing vessels with Ang1 for 30–45 min after exposure to pronase also attenuated the pronase-induced LP increase, when compared with pronase treatment alone (pronase alone: 3.6 ± 1.1-fold increase in LP over baseline (n = 4); pronase followed by Ang1: 1.5 ± 0.2-fold increase in LP over baseline (n = 5; P < 0.05, Mann–Whitney U test; Figure 3D).
3.5. Angiopoietin-1 modifies endothelial glycocalyx
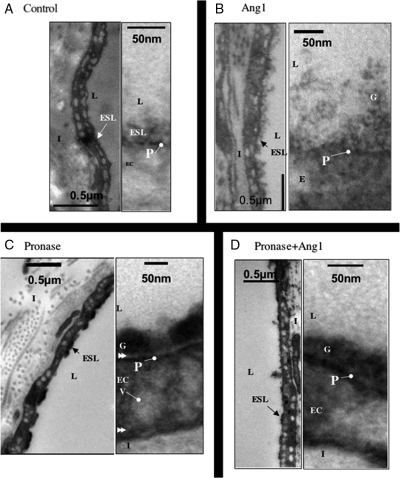
To visualize whether Ang1 modified the glycocalyx, the glycocalyx was stained in perfused vessels with Alcian Blue, and processed and examined by electron microscopy (Figure 4). Perfusion with Alcian Blue resulted in the staining of a 50–100 nm thick glycocalyx attached to, but above the plasmalemma (Figure 4A). Perfusion with Ang1 resulted in a thicker layer, which upon higher power examination could be seen to be a diffuse glycocalyx (Figure 4B) still attached to the plasmalemma. Treatment with pronase resulted in a darker stained glycocalyx (Figure 4C), which was clearly separated from the plasmalemma (arrows, Figure 4C). Subsequent treatment with Ang1 restored direct contact between glycocalyx and plasmalemma (Figure 4D).
Figure 4.
Angiopoietin-1 modifies endothelial glycocalyx in vivo. Electron micrographs of frog mesenteric microvessels following perfusion with (A) bovine serum albumin (BSA) alone, (B) Ang1, (C) pronase, and (D) Ang1 before and after pronase. Following perfusion with experimental agents, microvessels were treated with Alcian Blue (to stain endothelial glycocalyx), fixed with glutaraldehyde, and prepared for electron microscopy. Endothelial glycocalyx was significantly deeper following Ang1 treatment (B). Glycocalyx was shallower and more electron dense after exposure to pronase, and was separated from the plasmalemma (double arrow head) (C). Near-continuity between glycocalyx and plasmalemma, but not glycocalyx depth, was restored by Ang1 treatment of pronase-exposed vessels (D). ESL, endothelial surface layer; L, lumen; G, glycocalyx; P, plasmalemma; EC, endothelial cell; V, vesicle; I, interstitium.
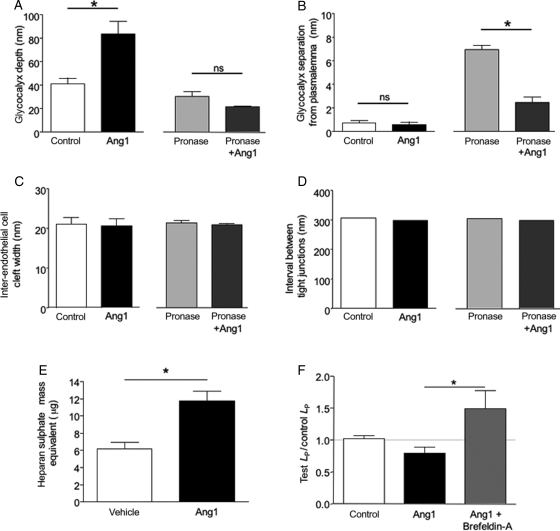
To quantify changes, the depth of endothelial glycocalyx in microvessels perfused with BSA alone, as determined with Alcian Blue staining and electron microscopic analysis, was measured. In control, the glycocalyx was 44.5 ± 3.6 nm (n = 96 measurements; n = 6 images, Figure 5A); glycocalyx depth in vessels perfused with Ang1 for 30 min was 87.1 ± 3.2 nm (n = 171 measurements; n = 6 images; P < 0.05, unpaired t-test; Figure 5A). Pronase exposure reduced the depth of glycocalyx to 31.7 ± 1.5 nm [n = 104 measurements, n = 20 images; control: 44.5 ± 3.6 nm; P < 0.05, one-way analysis of variance (ANOVA), Bonferroni]. In addition, pronase treatment elicited a significant separation of the glycocalyx from the underlying endothelial cell plasma membrane that was not evident under baseline conditions (6.9 ± 0.4 nm; Figure 5B; n = 104 measurements, n = 20 images). Ang1 treatment replenished this pronase-induced gap, immediately adjacent to the plasmalemma, with glycocalyx (separation 2.5 ± 0.4 nm; n = 66 measurements, n = 14 images; P < 0.05 vs. pronase alone, one-way ANOVA, Bonferroni, Figure 5B). However, total depth of glycocalyx was not preserved by Ang1 pre-treatment (21.4 ± 0.9 nm (n = 66 measurements, n = 14 images; not significantly different from pronase alone: P > 0.05, one-way ANOVA, Bonferroni). There was no difference in inter-endothelial cleft width between control [21.1 ± 1.6 nm (n = 6)] and Ang1 [20.6 ± 1.8 nm (n = 9)]-treated vessels (P > 0.8, unpaired t-test, Figure 5C). In addition, cleft lengths (control: 306 nm; Ang1: 299 nm) were similar in control and Ang1-treated vessels and the frequency of tight junctions within inter-endothelial clefts (approximately one per cleft for each group) was also the same resulting in no difference between tight junction interval along the cleft (Figure 5D). There were also no significant differences in inter-endothelial cleft width (pronase: 21.3 ± 0.59 nm; pronase+Ang1: 20.9 ± 0.4 nm; P > 0.05, one-way ANOVA, Bonferroni), inter-endothelial cleft length (pronase: 356 ± 46.0 nm; pronase+Ang1: 470 ± 111 nm; P > 0.35, unpaired t-test), or tight junction frequency [pronase: 1.2 tight junctions per cleft (n = 6); pronase+Ang1: 1.6 tight junctions per cleft (n = 7); P > ;0.45, unpaired t-test)], between pronase-treated and Ang1+pronase-treated vessels, nor between any of the treatment groups and baseline conditions (Figure 5C and D).
Figure 5.
Angiopoietin-1 modifies endothelial glycocalyx, but not inter-endothelial cleft ultrastructure. Inter-endothelial cleft and glycocalyx parameters were measured at regular 50 nm intervals in electron micrographs prepared from vessels perfused with control solution, angiopoietin-1, pronase, or pronase exposure in Ang1-perfused vessels. (A) Ang1 increased, and pronase decreased, glycocalyx depth. Ang1 perfusion did not alter glycocalyx depth in pronase-exposed vessels. (B) Pronase exposure caused separation of the glycocalyx from the plasmalemma; Ang1 reversed this effect (*P < 0.05, one-way analysis of variance). (C and D) There were no differences in the wide (C) or narrow (D) regions of inter-endothelial clefts between any experimental group. (E) Supernatant from human microvascular endothelial cells, cultured in the presence of 200 ng mL−1 Ang1 or vehicle for 48 h, was added to Alcian Blue solution. Ang1 treatment increased heparin sulphate equivalent (*P < 0.05, unpaired t-test). (F) The reduction in LP induced by Ang1 was abolished in the presence of the Golgi translocation inhibitor Brefeldin-A.
3.6. Angiopoietin-1 modifies endothelial cell glycosaminoglycan turnover, inhibition of which blocks the Ang1 reduction in LP
To determine whether Ang1 could affect the rate of endothelial glycocalyx turnover, endothelial cells in culture were treated with Ang1 in vitro. This increased the mass of glycosaminoglycan constituents in the supernatant of endothelial cells (e.g. heparan sulphate) from 6.2 ± 0.8 µg to 12.5 ± 1.1 µg (P < 0.05, unpaired t-test; Figure 5E). Glycocalyx is inserted onto the cell surface by vesicle fusion regulated by Golgi-mediated translocation. To determine whether this was required for the Ang1 effect in vivo, vessels were perfused with the Golgi vesicle translocation inhibitor brefeldin-A (100 µM) before treatment with Ang1. The reduction in baseline LP observed in response to Ang1 (Figure 1) was blocked by co-perfusing vessels with Ang1 (200 ng mL−1) and brefeldin A [Ang1 alone significantly lower than both control (P < 0.05) and Ang1+brefeldin-A (P < 0.05); brefeldin-A not significantly different from control (P > 0.05; all one-way ANOVA, Figure 5F)].
4. Discussion
We show here that perfusion of microvessels with continuous endothelium with Ang1 in vivo lowers baseline LP and increases reflection of albumin by the microvessel wall. This was demonstrated in non-inflamed vessels, in which LP and σ are the same as values previously reported for non-inflamed vessels in frogs27 and rats.28 The majority of reports demonstrating reduced transvascular solute movement following Ang1 treatment in vivo, or in intact organ preparations ex vivo, have either examined the effect of Ang1 following application of a stimulus (e.g. mustard oil29 or bradykinin10) or in animal models of disease (e.g. diabetic retinopathy6). Reduced solute movement may be related to: a change in the permeability coefficients of vessel walls (such as LP and σ); the extent of microvascular network perfusion;30 the net driving forces for solute movement (e.g. reduced hydrostatic pressure resulting from vasoconstriction); a combination of these factors; or to a number of other factors.13 The aforementioned studies did not discriminate between these factors. The results presented in this study are consistent with these reports, but show that one physiological mechanism for those observations is a direct effect of Ang1 on the permeability coefficients of microvascular walls.
We also show that Ang1 reduced the hydraulic conductivity–surface area product of the fenestrated glomerular capillary network, in agreement with reports of increased transendothelial electrical resistance (a marker of water and small solute permeability) across glomerular endothelial cell monolayers following Ang1 treatment.15 These observations together highlight three actions of Ang1 on microvascular permeability coefficients: reduced LP of continuous microvessels; increased albumin reflection by continuous microvessels; and reduced LPA of fenestrated microvessels. The pathways that regulate these permeability coefficients have distinct components, and this diversity can provide insight into the cellular mechanisms underlying the Ang1 effect.
The frequency of breaks in the tight junction strands in inter-endothelial clefts are a critical determinant of the LP of continuous microvessels. Substances that increase the number of tight junction strands present in inter-endothelial clefts (e.g. cAMP analogues) reduce baseline LP.31 Ang1 modifies a number of inter-endothelial cleft molecules, e.g. occludin, PECAM-1, β-catenin, and VE-cadherin.14,32 These tight-junction strand breaks, however, are not significant regulators of the reflection coefficient of the vessel wall,31 and changes in inter-endothelial clefts are therefore unlikely to explain the increase in σ observed following application of Ang1. The cellular structure that correlates to the ‘large pore’ that governs the magnitude of σ remains controversial: both trans-endothelial (vesiculo-vacuolar organelles, caveolae), and inter-endothelial pathways have been postulated.17 Furthermore, reduced LPA in response to Ang1 in the fenestrated glomerular capillary network is unlikely to represent alterations in hydraulic inter-endothelial pathways. The vast majority of fluid flux across the glomerular endothelial cell layer is via fenestrae, rather than inter-endothelial clefts,33 such that it seems highly unlikely that Ang1 reduces glomerular LPA by altering the clefts between glomerular endothelial cells.
It is conceivable that the plethora of observed actions of Ang1 on permeability coefficients are mediated by modification of a variety of different ultrastructural features of different microvascular beds that result in parallel changes in permeability coefficients. An alternative explanation is that Ang1 modifies a structure that is common to both continuous and fenestrated capillaries, and that contributes to both hydraulic conductivity and molecular sieving of microvessel walls. The endothelial glycocalyx appears to be a good candidate for this. The fibre–matrix junction break model, originally outlined by Curry and Michel in 1980,34 proposes that the size, orientation and spacing of the fibres that form the endothelial glycocalyx layer on the luminal aspect of endothelial cells contribute to the resistance to water flow and molecular selectivity of the vessel wall. Endothelial glycocalyx lies across the surface of both inter-endothelial clefts in vessels with continuous endothelium21 and across fenestrae in glomerular capillaries,35 and endothelial glycocalyx has been shown to contribute to hydraulic resistance and molecular sieving in vessels with continuous endothelia21,36 and vessels with fenestrated endothelia.37,38 Modification of a number of features of glycocalyx by Ang1, such as depth and/or density, would be expected to increase the hydraulic resistance (i.e. decrease LP or LPA) and to increase reflection of large solutes (i.e. increase σ).17 Ang1-induced changes in microvascular glycocalyx depth, and endothelial cell supernatant glycosaminoglycan content, support the contention that Ang1 modifies endothelial glycocalyx.
We have shown an increase in the depth of glycocalyx lining microvascular endothelial cells following perfusion with Ang1 for 30 min in vivo. The ultrastructural changes described here induced by Ang1 would be predicted to alter microvascular permeability characteristics (given the contribution that endothelial glycocalyx makes to the magnitude of permeability coefficients21,36,37), but these observations do not by themselves demonstrate that the Ang1-induced change in permeability coefficients is mediated by an alteration in endothelial glycocalyx. However, taken together with the loss of the Ang1-induced reduction in LP by inhibition of the Golgi vesicle translocation system upon which translocation of glycosaminoglycans to the cell surface depends;22,39 both abrogation and accelerated recovery from the rise in LP that accompanies removal of the endothelial glycocalyx in vivo and that the prevention of pronase-induced increased LP was accompanied by restoration of near-continuity between the glycocalyx and the endothelial plasmalemma when the microvessels were pre-treated with Ang1, these findings strongly implicate the glycocalyx as being a key mechanism through which Ang1 exerts its permeability effects. Extracellular matrix modulates the activity and availability of endothelial cell survival factor pathways:40 our observations indicate that this interaction is reciprocal, and that endothelial cell survival factors can also modulate extracellular matrix components, such as those in the endothelial glycocalyx. These observations do not preclude the possibility that Ang1 is also affecting other aspects of endothelial cell biology such as the density and activity of inter-endothelial junction molecules, thereby making a parallel contribution to the observed changes in microvascular barrier properties, particularly in vessels with continuous endothelium.
Ang1 has heparin-binding/extracellular matrix-binding properties,40 and could modify the barrier properties endowed by the endothelial glycocalyx by altering glycocalyx biochemical structure. The receptors and downstream signalling pathways responsible for this effect represent an opportunity for further investigation. The time-course for the Ang1-induced changes in glycocalyx effects (permeability coefficients and glycocalyx thickness both changed within 30 min of exposure) indicate that synthesis of de novo glycocalyx constituents is unlikely to be responsible. Ang1 is capable of altering the cell surface expression of other molecules over a similar time-course (e.g. Tie240), and microtubule-dependent trafficking of heparan sulphate has been observed in polarised renal epithelial cells.41 Translocation of glycocalyx constituents from stores within endothelial cells to the plasmalemma may therefore explain the observed phenomena. We support this contention by demonstrating that inhibiting the translocation of Golgi vesicles, which transport glycosaminoglycans (e.g. hyaluronan22) to the cell surface, inhibited the effect of Ang1 on LP. Brefeldin-A disrupts translocation of cellular material in minutes39 and this is in keeping with our observations of altered LP and glycocalyx depth over this period.
The observations that Ang1 increased glycocalyx depth and decreased LP accords with theoretical predictions that the hydraulic resistance of the glycocalyx is determined by its depth. However, a change in the molecular constitution or arrangement of the glycocalyx is necessary to explain any change in molecular sieving,33 and Ang1 may therefore translocate different glycocalyx constituents, or glycocalyx constituents in different proportions, from those observed under baseline conditions. Different effects of Ang1 on different aspects of endothelial cell biology may occur in parallel (e.g. restoration of continuity between glycocalyx and plasmalemma at the endothelial cell surface, and changes in inter-endothelial cleft molecules), and may contribute to the multiplicity of changes in permeability coefficients observed in different types of microvessels in these studies.
In summary, we show for the first time that Ang1 alters microvascular permeability coefficients of intact microvessels under baseline conditions in vivo. This provides a physiological mechanism for previous reports that Ang1 alters transmural flux of solutes and tracers. We present anatomical, biochemical, and physiological evidence for Ang1-induced modification of a structure that is common to both continuous and fenestrated endothelia—the endothelial glycocalyx—and suggest that these modifications contribute to the Ang1-induced changes in microvascular barrier function.
Supplementary Material
Supplementary Material is available at Cardiovasular Research online.
Funding
This work was supported by The Wellcome Trust (grant number 69134 to A.H.J.S., 77589 to C.A.G.), the MRC (GR0600920 to C.R.N.), the Richard Bright VEGF Research Trust, and the British Heart Foundation (FS/07/059/24071 to L.M.S., grant number BB2000003 and BS/06/005 to D.O.B.). Funding to pay the Open Access publication charges for this article was provided by the Wellcome Trust.
Conflict of interest: None declared.
References
- 1.Brindle NP, Saharinen P, Alitalo K. Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. 2006;98:1014–1023. doi: 10.1161/01.RES.0000218275.54089.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Satchell S, Harper S, Mathieson P. Angiopoietin-1 is normally expressed by periendothelial cells. Thromb Haemost. 2001;86:1597–1598. [PubMed] [Google Scholar]
- 3.Wong A, Haroon Z, Werner S, Dewhirst M, Greenberg C, Peters K. Tie2 expression and phosphorylation in angiogenic and quiescent adult tissues. Circ Res. 1997;81:567–574. doi: 10.1161/01.res.81.4.567. [DOI] [PubMed] [Google Scholar]
- 4.Nagamine K, Furue M, Fukui A, Asashima M. Induction of cells expressing vascular endothelium markers from undifferentiated Xenopus presumptive ectoderm by co-treatment with activin and angiopoietin-2. Zool Sci. 2005;22:755–761. doi: 10.2108/zsj.22.755. [DOI] [PubMed] [Google Scholar]
- 5.Iwama A, Hamaguchi I, Hashiyama M, Murayama Y, Yasunaga K, Suda T. Molecular cloning and characterisation of mouse Tie and Tek receptor tyrosine kinase genes and their expression in haematopoietic stem cells. Biochem Biophys Res Commun. 1993;195:301. doi: 10.1006/bbrc.1993.2045. [DOI] [PubMed] [Google Scholar]
- 6.Joussen A, Poulaki V, Tsujikawa A, Qin W, Qaum T, Xu Q, et al. Supression of diabetic retinopathy with angiopoietin-1. Am J Pathol. 2002;160:1683–1693. doi: 10.1016/S0002-9440(10)61115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, et al. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282:468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 8.Thurston G, Wang Q, Baffert F, Rudge J, Papadopoulos N, Jean-Guillaume D, et al. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 9.Thurston G, Suri C, Smith K, McClain J, Sato T, Yancopoulos G, et al. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 10.Baffert F, Le T, Thurston G, McDonald DM. Angiopoietin-1 decreases plasma leakage by reducing number and size of endothelial gaps in venules. Am J Physiol Heart Circ Physiol. 2006;290:H107–H118. doi: 10.1152/ajpheart.00542.2005. [DOI] [PubMed] [Google Scholar]
- 11.Roviezzo F, Tsigkos S, Kotanidou A, Bucci M, Brancaleone V, Cirino G, et al. Angiopoietin-2 causes inflammation in vivo by promoting vascular leakage. J Pharmacol Exp Ther. 2005;314:738–744. doi: 10.1124/jpet.105.086553. [DOI] [PubMed] [Google Scholar]
- 12.Hall E, Brookes ZL. Angiopoietin-1 increases arteriolar vasoconstriction to phenylephrine during sepsis. Regul Pept. 2005;131:34–37. doi: 10.1016/j.regpep.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Bates DO, Lodwick D, Williams B. Vascular endothelial growth factor and microvascular permeability. Microcirculation. 1999;6:83–96. [PubMed] [Google Scholar]
- 14.Gamble JR, Drew J, Trezise L, Underwood A, Parsons M, Kasminkas L, et al. Angiopoietin-1 is an antipermeability and anti-inflammatory agent in vitro and targets cell junctions. Circ Res. 2000;87:603–607. doi: 10.1161/01.res.87.7.603. [DOI] [PubMed] [Google Scholar]
- 15.Satchell SC, Anderson KL, Mathieson PW. Angiopoietin 1 and vascular endothelial growth factor modulate human glomerular endothelial cell barrier properties. J Am Soc Nephrol. 2004;15:566–574. doi: 10.1097/01.asn.0000115397.22519.03. [DOI] [PubMed] [Google Scholar]
- 16.Curry FR. Microvascular solute and water transport. Microcirculation. 2005;12:17–31. doi: 10.1080/10739680590894993. [DOI] [PubMed] [Google Scholar]
- 17.Michel CC, Curry FE. Microvascular permeability. Physiol Rev. 1999;79:703–761. doi: 10.1152/physrev.1999.79.3.703. [DOI] [PubMed] [Google Scholar]
- 18.Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:F579–F596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- 19.Hillman NJ, Whittles CE, Pocock TM, Williams B, Bates DO. Differential effects of vascular endothelial growth factor-C and placental growth factor-1 on the hydraulic conductivity of frog mesenteric capillaries. J Vasc Res. 2001;38:176–186. doi: 10.1159/000051044. [DOI] [PubMed] [Google Scholar]
- 20.Salmon AH, Neal CR, Bates DO, Harper SJ. Vascular endothelial growth factor increases the ultrafiltration coefficient in isolated intact Wistar rat glomeruli. J Physiol. 2006;570:141–156. doi: 10.1113/jphysiol.2005.099184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamson RH. Permeability of frog mesenteric capillaries after partial pronase digestion of the endothelial glycocalyx. J Physiol. 1990;428:1–13. doi: 10.1113/jphysiol.1990.sp018197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wann AK, Ingram KR, Coleman PJ, McHale N, Levick JR. Mechanosensitive hyaluronan secretion: stimulus-response curves and role of transcriptiond-translation-translocation in rabbit joints. Exp Physiol. 2009;94:350–361. doi: 10.1113/expphysiol.2008.045203. [DOI] [PubMed] [Google Scholar]
- 23.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 24.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 25.Gold EW. The quantitative spectrophotometric estimation of total sulfated glycosaminoglycan levels. Formation of soluble Alcian Blue complexes. Biochim Biophys Acta. 1981;673:408–415. doi: 10.1016/0304-4165(81)90472-4. [DOI] [PubMed] [Google Scholar]
- 26.Michel CC, Mason JC, Curry FE, Tooke JE, Hunter PJ. A development of the Landis technique for measuring the filtration coefficient of individual capillaries in the frog mesentery. Q J Exp Physiol Cogn Med Sci. 1974;59:283–309. doi: 10.1113/expphysiol.1974.sp002275. [DOI] [PubMed] [Google Scholar]
- 27.Michel CC. Filtration coefficients and osmotic reflexion coefficients of the walls of single frog mesenteric capillaries. J Physiol. 1980;309:341–355. doi: 10.1113/jphysiol.1980.sp013512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kendall S, Michel CC. The measurement of permeability in single rat venules using the red cell microperfusion technique. Exp Physiol. 1995;80:359–372. doi: 10.1113/expphysiol.1995.sp003853. [DOI] [PubMed] [Google Scholar]
- 29.Thurston G, Rudge J, Ioffe E, Zhou H, Ross L, Croll S, et al. Angiopoietin-1 protects the adult vasculature against plasma leakage. Nat Med. 2000;6:460–463. doi: 10.1038/74725. [DOI] [PubMed] [Google Scholar]
- 30.Benest AV, Salmon AH, Wang W, Glover CP, Uney J, Harper SJ, et al. VEGF and angiopoietin-1 stimulate different angiogenic phenotypes that combine to enhance functional neovascularization in adult tissue. Microcirculation. 2006;13:423–437. doi: 10.1080/10739680600775940. [DOI] [PubMed] [Google Scholar]
- 31.Adamson RH, Liu B, Fry GN, Rubin LL, Curry FE. Microvascular permeability and number of tight junctions are modulated by cAMP. Am J Physiol. 1998;274:H1885–H1894. doi: 10.1152/ajpheart.1998.274.6.H1885. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Pampou S, Fujikawa K, Varticovski L. Opposing effect of angiopoietin-1 on VEGF-mediated disruption of endothelial cell-cell interactions requires activation of PKC beta. J Cell Physiol. 2004;198:53–61. doi: 10.1002/jcp.10386. [DOI] [PubMed] [Google Scholar]
- 33.Levick JR. Flow through interstitium and other fibrous matrices. Q J Exp Physiol. 1987;72:409–437. doi: 10.1113/expphysiol.1987.sp003085. [DOI] [PubMed] [Google Scholar]
- 34.Curry FE, Michel CC. A fiber matrix model of capillary permeability. Microvasc Res. 1980;20:96–99. doi: 10.1016/0026-2862(80)90024-2. [DOI] [PubMed] [Google Scholar]
- 35.Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvasc Res. 1997;53:1–13. doi: 10.1006/mvre.1996.1987. [DOI] [PubMed] [Google Scholar]
- 36.Adamson RH, Lenz JF, Zhang X, Adamson GN, Weinbaum S, Curry FE. Oncotic pressures opposing filtration across non-fenestrated rat microvessels. J Physiol. 2004;557:889–907. doi: 10.1113/jphysiol.2003.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jeansson M, Haraldsson B. Morphological and functional evidence for an important role of the endothelial cell glycocalyx in the glomerular barrier. Am J Physiol Renal Physiol. 2006;290:F111–F116. doi: 10.1152/ajprenal.00173.2005. [DOI] [PubMed] [Google Scholar]
- 38.Singh A, Satchell SC, Neal CR, McKenzie EA, Tooke JE, Mathieson PW. Glomerular endothelial glycocalyx constitutes a barrier to protein permeability. J Am Soc Nephrol. 2007;18:2885–2893. doi: 10.1681/ASN.2007010119. [DOI] [PubMed] [Google Scholar]
- 39.Rilla K, Siiskonen H, Spicer AP, Hyttinen JM, Tammi MI, Tammi RH. Plasma membrane residence of hyaluronan synthase is coupled to its enzymatic activity. J Biol Chem. 2005;280:31890–31897. doi: 10.1074/jbc.M504736200. [DOI] [PubMed] [Google Scholar]
- 40.Fukuhara S, Sako K, Minami T, Noda K, Kim HZ, Kodama T, et al. Differential function of Tie2 at cell-cell contacts and cell-substratum contacts regulated by angiopoietin-1. Nat Cell Biol. 2008;10:513–526. doi: 10.1038/ncb1714. [DOI] [PubMed] [Google Scholar]
- 41.De Almeida JB, Stow JL. Disruption of microtubules alters polarity of basement membrane proteoglycan secretion in epithelial cells. Am J Physiol. 1991;261:C691–C700. doi: 10.1152/ajpcell.1991.261.1.C691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.