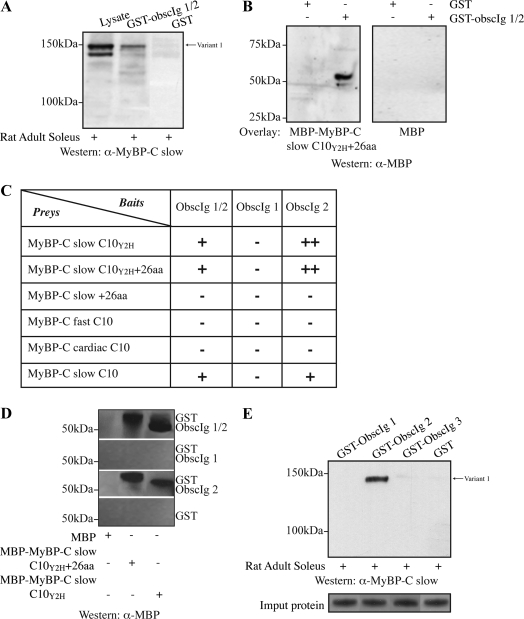
Figure 6.
Characterization of the interaction between the NH2 terminus of obscurin and the COOH terminus of MyBP-C slow variant-1. (A) Equivalent amounts of GST-Obscurin-Ig1/2 and GST were bound to glutathione matrices and incubated with protein homogenates prepared from adult rat soleus muscle. Total lysates were also included in the Western blot for comparison purposes. Recombinant GST-Obscurin-Ig1/2, but not GST, efficiently adsorbed native MyBP-C slow variant-1 (top band), and to a lesser degree, variant-4 (bottom band), as shown by SDS-PAGE and immunoblotting with the MyBP-C slow antibody. (B) The specific and direct interaction between the NH2 terminus of obscurin and the COOH terminus of MyBP-C slow variant-1 was further verified in vitro by overlay assays. Equal amounts of bacterially expressed GST and GST-obscurin-Ig1/2 were separated by SDS-PAGE and overlaid with recombinant MBP-MyBP-C slow-C10Y2H+26aa or control MBP. MBP-MyBP-C slow-C10Y2H+26aa, but not MBP, bound to GST-Obscurin-Ig1/2, but not to GST. (C) The minimal regions of obscurin and MyBP-C slow variant-1 involved in binding were identified using the yeast system. A series of deletion constructs were generated for both obscurin and MyBP-C slow variant-1 and introduced into the bait and prey vector, respectively. Yeast two-hybrid analysis indicated that the Ig2 domain of obscurin and the C10 domain of MyBP-C slow are both necessary and sufficient to support their direct association in vitro. This interaction is specific for the C10 domain of MyBP-C slow because neither the C10 domain of the fast isoform nor the C10 domain of the cardiac isoform were able to interact with the NH2 terminus of obscurin in the yeast system. (D) To confirm the ability of the minimal interacting domains of obscurin and MyBP-C slow variant-1 identified in the yeast system to support their direct association, we also used an in vitro pull-down assay. Equivalent amounts of bacterially expressed GST-Obscurin-Ig1/2, GST-Obscurin-Ig1, GST-Obscurin-Ig2, and GST were bound to glutathione matrices and tested for their ability to retain recombinant MBP, MBP-MyBP-C slow-C10Y2H+26aa, and MBP-MyBP-C slow C10Y2H in a pull-down assay. GST-Obscurin-Ig1/2 and GST-Obscurin-Ig2, but not GST-Obscurin-Ig1 or GST, were able to efficiently pull down MBP-MyBP-C slow-C10Y2H+26 and MBP-MyBP-C slow-C10Y2H. (E) GST fusion proteins of obscurin (GST-Obscurin-Ig1, GST-Obscurin-Ig2, and GST-Obscurin-Ig3) were incubated with protein homogenates prepared from adult rat soleus muscle and examined for their ability to adsorb endogenous MyBP-C slow variant-1; GST-Obscurin-Ig2 adsorbed native MyBP-C slow variant-1, whereas neither GST-Obscurin-Ig1 nor GST-Obscurin-Ig3 was able to precipitate endogenous MyBP-C slow variant-1. Coomassie Blue staining of the input protein is shown in the bottom panel to indicate equal loading.