Figure 7.
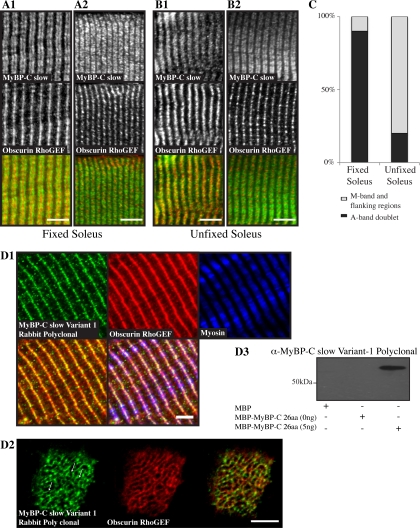
MyBP-C slow variant-1 preferentially accumulates at the M-band. Fixed (A1 and A2) and unfixed (B1 and B2) adult rat soleus fibers were costained with antibodies specific for the MyBP-C slow isoforms (A1–B2, top, green) and the Rho-GEF domain of obscurin (A1–B2, middle, red), which specifically labels M-bands. (A1 and A2) In ∼90% of myofibers in fixed soleus muscle, MyBP-C slow assumed its typical staining at A-bands (top) and obscurin at M-bands (middle), as clearly shown in the overlay (bottom). In ∼10% of myofibers, however, MyBP-C slow was also present midway of A-bands, at M-bands (top), as shown by colabeling with antibodies to the Rho-GEF domain of obscurin (middle); areas of overlap between MyBP-C slow and obscurin look yellow in the overlay (bottom). (B1 and B2) By contrast, in unfixed soleus muscle, in ∼80% of myofibers the MyBP-C slow antibody stained the entire A-band, including the central M-band (top), as shown by costaining with antibodies to the Rho-GEF domain of obscurin (middle); areas of coincident distribution between MyBP-C slow and obscurin look yellow in the overlay (bottom). In the remaining ∼20% of myofibers, MyBP-C slow assumed its typical organization at A-bands (top) as no overlapping staining with obscurin (middle) was observed in the color overlay (bottom). Bar (A and B), 10 μm. (C) Graph presenting the percentage of cells showing labeling at A- or A- and M-bands in fixed and unfixed soleus muscle. (D1) Labeling of rat adult soleus muscle with affinity-purified rabbit antibodies raised against the unique COOH terminus of MyBP-C slow-variant-1 (top left, green) and antibodies to the Rho-GEF domain of obscurin (top middle, red) that label the M-band and to the slow isoform of sarcomeric myosin (top right, blue) revealed the presence of MyBP-C slow variant-1 in the middle of A-bands (bottom right, triple staining overlay), at M-bands and possibly flanking regions (bottom left, double staining overlay). (D2) Cross sections of adult rat soleus muscle were costained with antibodies to the unique COOH terminus of MyBP-C slow variant-1 (left, green) and the Rho-GEF domain of obscurin (middle, red). Similarly to obscurin, MyBPC slow variant-1 is present in a reticular pattern at the level of the M-band, as shown in the color overlay (right; and arrows). Bar, 5 μm. (D3) Western blot analysis indicated that the affinity-purified antibodies for MyBP-C slow variant-1, used in D1, specifically recognize recombinant MBP-MyPB-C-26aa.