Fig. 1.
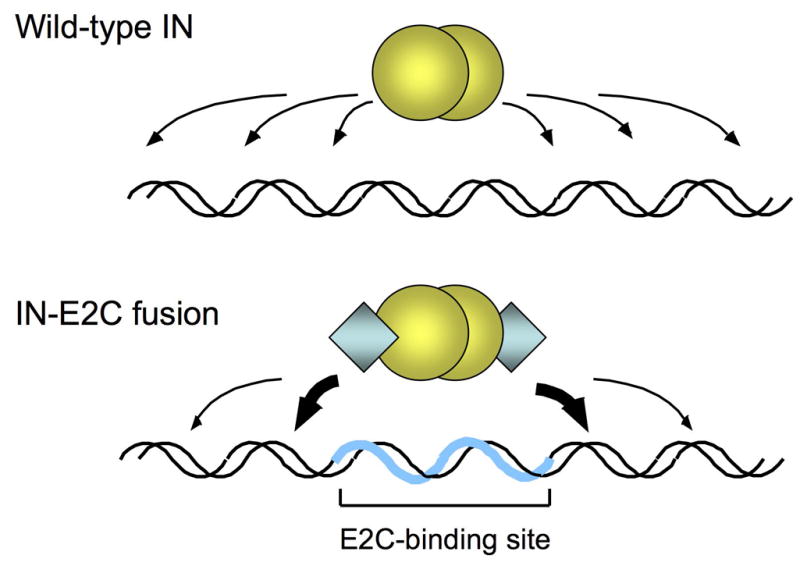
Site-directed integration catalyzed by engineered IN fusion proteins. Integration of retroviral DNA catalyzed by wild-type IN (yellow spheres) is largely non-specific and can occur at various positions along the length of a target DNA (upper panel). In reactions catalyzed by fusion proteins consisting of IN and the designed polydactyl zinc-finger protein E2C (blue diamonds; lower panel), the fusion protein binds specifically to the E2C-recognition sequence e2c (thick blue line) and thereby biases integration in the nearby regions (thick arrows). The e2c site is non-palindromic, and most of the amino acid-DNA contacts are made with the G-rich strand of the target sequence [34, 37]. The IN is depicted as a dimer, but the multimeric state of active IN has not been firmly established.
