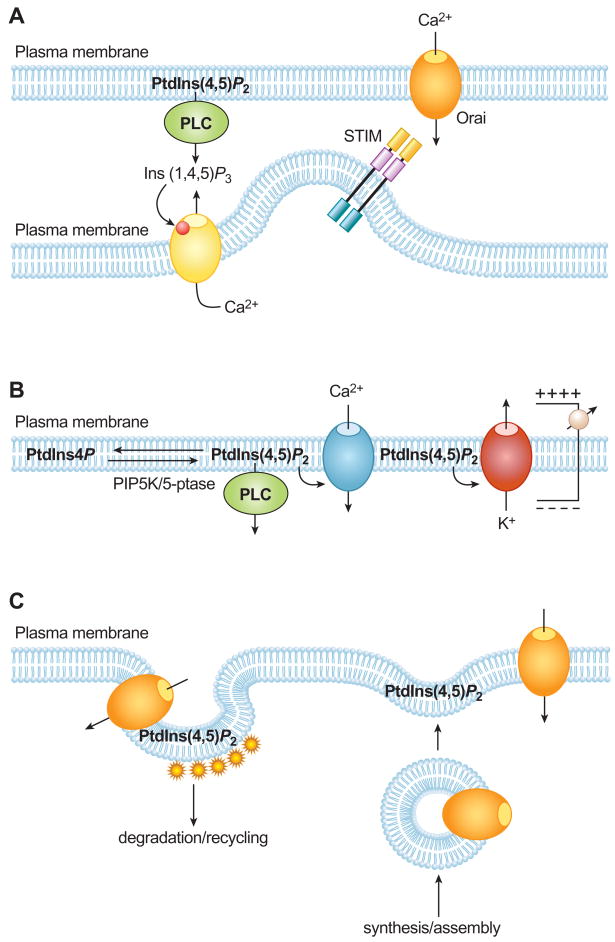
Figure 1.
Different forms of regulation of ion channels relevant to Ca2+ signaling. (A) In the canonical phosphoinositide signaling pathway, phospholipase C (PLC) activation from cell surface receptors leads to the generation of Ins(1,4,5)P3, which, in turn, releases Ca2+ from the endoplasmic reticulum (ER) Ca2+ stores via Ins(1,4,5)P3 receptor-channels. The decreased ER luminal Ca2+ is sensed by the ER resident STIM proteins that respond with oligomerization and interaction with PM (PM) Orai/CRACM channels at ER-PM junctional sites activating Ca2+ influx. This form of Ca2+ entry is indirectly linked to Ins(1,4,5)P3 production, but direct regulation of the Orai channels and possibly STIM interaction with the PM by phosphoinositides has also been proposed. (B) Direct regulation of ion channel (and transporter) activities by PM phosphoinositides. Here the lipid directly interacts with some molecular component of the channel usually by electrostatic interaction involving positively charged regions of the channel facing the cytoplasm. Phosphoinositide levels can change as a result of PLC activation, or due to a shift in the balance of 5-phosphatase and PIP 5-kinase ctivities. Channels with the highest affinity to inositides may not respond to decreasing inositide levels if they tightly hold on to their bound lipid, therefore, the same lipid change can evoke a variety of activity changes depending on the lipid affinities of the channels. Potassium channels are also known to be sensitive to phosphoinositide changes and because they have significant impact on the membrane potential, they can indirectly affect Ca2+ entry by changing the electrochemical driving force of Ca2+. This is especially important for voltage-gated Ca2+ channels or for the inwardly rectifying ICRAC channels. (C) Ion channels are also moved around within the cells as a result of membrane trafficking. The synthesis and assembly of channels usually occures in the ER and the channels are glycosylated in the Golgi from where they are heading their final destination, the PM. It is now believed that most channels are not active within the internal membranes, although there are examples suggesting otherwise. PtdIns(4,5)P2 is present in the PM and channels and transporters that require this lipid for activity gain functionality only upon insertion in the PM. In the opposite process, active channels can be removed from the PM by various interanlization routes, some clathrin-dependent, some –independent. Internalized channles are moved into recycling compartments from where they can recycle back to the membrane or head to degradation. All of these trafficking events are regulated by phosphoinositides.