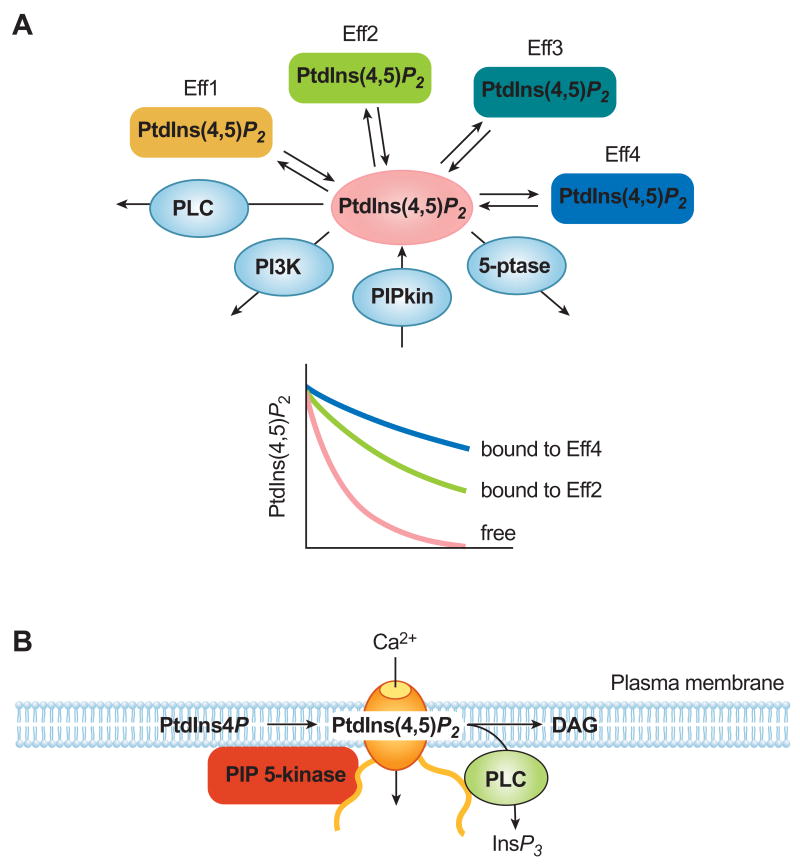
Figure 2.
Principles governing the compartmentalized regulation of ion channels (or other effectors) by phosphoinositides. (A) PtdIns(4,5)P2 in the plasma membrane exists in free form but is also bound to several proteins regulated by this lipid (effectors, Eff1-4). These could be channels, transporters, actin binding proteins, clathrin adaptors, enzymes and several other proteins. The enzymes that produce and convert these lipids [including PIP 5-kinases (PIPkin), phosphoinositide 5-phosphatases (5-ptase), Class I PI 3-kinases (PI3K) and phospholipase C (PLC) enzymes] act on the unbound fraction (in pink) which is in a dynamic equilibrium with the protein bound fractions (Eff1-4). Changes in the level of free PtdIns(4,5)P2 are reflected differently in the pools bound to the various effectors depending on their rates of dissociation. This represents a functional compartmentalization that does not necessarily mean a phisical segregation but may result in different metabolic turnover rates. (B) Theoretical example of the regulation of an effector (ion channel) by a phosphoinositide dedicated to the protein. Here the associated kinase or phosphatase ensures that the phosphoinositide is not diffusing away from the effector and does not contribute to the larger “shared” pool of the inositol lipid in question.